Solid carcinoma. Malignant tumors
Currently, there is no comprehensive classification of tumors, since there are a number of controversial unresolved problems, such as the histogenesis of tumors, the origin of a number of normal cells, tissue structures that can be the source of a tumor. It is still controversial, for example, the origin of some elements of the hematopoietic system, a number of structures that have the ability to produce biologically active substances (APUD system, glomus cells, vascular pericytes, pigment cells, interstitial cells of transversely striated muscles, renal medulla, etc.).
Source
Epithelium: flat and transitional
prismatic and ferruginous
Stem cells and epithelial progenitor cells
The General Nomenclature of Human Tumors published by WHO in 1959 was indicative in nature. It was based on histogenetic and localization principles, taking into account the clinical course of the disease. Subsequently, as an annex to ICD-9, an expanded nomenclature of tumors was created, which formed the basis for the WHO tumor classifications. The International Classification of Tumors allows comparison of materials obtained in different countries; it satisfies the needs of clinical and anatomical analysis of tumors and differential diagnosis.
Within the framework of WHO classifications, clinicians use an additional classification according to the TNM system (T - tumor, N - metastases to lymph nodes, M - hematogenous metastases). This classification gives a clear idea of the local spread of the tumor, as well as the phase of the tumor process, which is of great importance for prognosis and therapeutic purposes.
Principles of morphological classification
Based on the histogenetic principle, taking into account the morphological structure, localization, structural features in individual organs, benignity and malignancy, 7 groups of tumors were identified.
1. Epithelial tumors without specific localization (organ-nonspecific).
2. Tumors of exo- and endocrine glands, as well as epithelial integuments (organ-specific).
3. Mesenchymal tumors.
4. Tumors of melanin-forming tissue.
5. Tumors of the nervous system and meninges.
6. Tumors of the blood system.
7. Teratomas.
It should be noted that the division of epithelial tumors into organ-nonspecific and organ-specific is not justified, since tissue organ-specific markers have been found for most tumors, which are of decisive importance in the morphological diagnosis of tumors. In the following presentation we will consider the most common groups.
Epithelial tumors without specific localization
These tumors, developing from squamous or glandular epithelium that does not perform a specific function, are divided into benign and malignant.
Benign tumors
This group of epithelial tumors includes papilloma and adenoma.
Papilloma. Tumor of squamous or transitional epithelium. It usually has a papillary appearance (resembles cauliflower), is built from cells of the growing integumentary epithelium, and the number of layers is increased. The stroma is well expressed and grows together with the epithelium. Papilloma retains the properties of epithelial tumors: polarity, complexity and the presence of a basement membrane. Most often, papilloma is observed on the skin, mucous membranes of the oral cavity, esophagus, vocal cords, renal pelvis, and bladder ureters.
Adenoma. Tumor of prismatic and glandular epithelium. It is found on mucous membranes lined with prismatic epithelium and in glandular organs. Adenomas of the mucous membranes that protrude above the surface in the form of a polyp are called adenomatous (glandular) polyps. If the stroma is highly developed in the adenoma, then they speak of fibroadenoma. In a tumor, the epithelium maintains complexity, polarity, and a basement membrane. There are alveolar, trabecular, and papillary adenomas. If cavities form in the adenoma, then they speak of cystadenoma.
Malignant tumors
A malignant tumor that develops from poorly differentiated epithelial cells is cancer. The following microscopic forms of cancer are distinguished.
Hepatocellular adenoma. A benign tumor arising from hepatocytes forms trabeculae.
Hepatocellular (hepatocellular) cancer. Constructed from typical hepatocytes. It can grow in the form of one or several nodes. Usually builds trabeculae, less often - tubular structures. The tumor stroma is poorly expressed, there are many thin-walled vessels.
Adenoma. A benign tumor has a tubular, sometimes trabecular structure. Depending on the composition of the cells, dark cell, light cell (hypernephroid) and acidophilic adenomas are distinguished.
"Cancer in situ" (carcinoma in situ). A form of cancer without infiltrating growth, but with pronounced cellular atypia. The tumor grows within the epithelial layer.
Squamous cell (epidermal) cancer. Develops in the skin and mucous membranes covered with squamous epithelium. The tumor epithelium loses polarity, complexity and basement membrane. As a result of keratinization (keratinizing cancer), cancerous pearls are formed. In epithelial cells with low differentiation, keratinization does not occur. Nonkeratinizing squamous cell carcinoma develops.
Adenocarcinoma (glandular cancer). It develops from the prismatic epithelium lining the mucous membranes and the epithelium of the glands. The tumor exhibits cellular atypia, the epithelium loses its complexity, polarity, and basement membrane. Depending on the degree of differentiation, highly differentiated, moderately differentiated and poorly differentiated adenocarcinoma are distinguished. Necrosis and hemorrhage are common in the tumor.
Mucous (colloid) cancer. The cells that make up the tumor produce large amounts of mucus and have signs of pronounced cellular atypia. Mucosal cancer is one of the forms of poorly differentiated adenocarcinoma.
Solid cancer. A form of poorly differentiated cancer. Cellular atypia is pronounced in the cells. It has a trabecular structure.
Small cell cancer. A form of poorly differentiated cancer. Constructed of lymphocyte-like cells. Necrosis and hemorrhage are often observed in it.
Fibrous cancer (scirrhus). Constructed of atypical cells embedded in a highly developed stroma (coarse fibrous connective tissue). Refers to poorly differentiated cancers.
Medullary (adenogenic) cancer. A form of poorly differentiated cancer, built from atypical epithelial cells. Characterized by a predominance of parenchyma over stroma. It often contains necrosis and hemorrhage.
Tumors of exo- and endocrine glands, as well as epithelial integuments
The cells of these tumors, preserving the functional and morphological features of the organs from which they develop, are dispersed in the epithelial integument, exo- and endocrine glands.
Source:
Liver - hepatocytes
Kidney - tubular epithelium Metanephrogenic tissue
Mammary gland epithelium of alveoli and excretory ducts epithelium of large ducts (localized in the area of the nipple and areola)
Uterus - chorion membrane
Epithelium of sweat gland ducts
Epithelium of the secretory sections of the sweat glands, epithelium of the hair follicles
Epithelium of various parts of skin appendages
Tumors - malignant
Hepatocellular carcinoma
Renal cell carcinoma Nephroblastoma
"Cancer in place": lobular, ductal
Paget's disease (cancer)
Destructive (malignant) hydatidiform mole; chorionepithelioma (chorionic carcinoma)
Basal cell carcinoma
Renal cell (hypernephroid) cancer. A malignant tumor, built from atypical cells, is often accompanied by necrosis and hemorrhage. Characterized by growth along the veins and early hematogenous metastases to the lungs, bones, liver, and opposite kidney. Depending on the cellular composition, the following microscopic forms are distinguished: clear cell, granular cell, glandular, sarcoma-like, mixed cell.
Nephroblastoma (embryonic kidney cancer, Wilms tumor). A malignant tumor of mixed structure, consists of epithelial cells forming solid and tubular structures, and striated muscles, fatty tissue, cartilage, and blood vessels. Occurs in children.
BREAST.
Tumors are very diverse and often develop against the background of dishormonal benign dysplasia.
Fibroadenoma. A benign tumor of glandular epithelium with a highly developed stroma. There are pericanular and intracanalicular fibroadenomas. Leaf-shaped (phylloid) tumors are rare. Breast cancer. It is represented by the following forms: non-infiltrating lobular and intraductal cancer, Paget's disease.
Non-infilating lobular cancer (lobular “carcinoma in situ”). Constructed from atypical cells, grows in a lobule, has glandular or solid variants.
Non-infiltrating intraductal carcinoma (ductal “carcinoma in situ”). It can be papillary, cribriform and acne-like. It grows within the duct, often undergoes necrosis, and calcifications are possible.
Paget's disease. Develops from the epidermis or epithelial cells of large ducts. Large light cells (Paget's cells) are formed in the basal and middle layers of the epidermis. The tumor is localized in the area of the nipple and areola.
All of these forms of breast cancer, when progressing, turn into infiltrating breast cancer.
Epithelial tumors are represented by destructive hydatidiform mole and chorionepithelioma.
Destructive (malignant) hydatidiform mole. It is represented by large chorionic villi, growing into the walls of the veins of the uterus and small pelvis. Syncytial cells predominate in the villi. Sometimes it is very difficult to distinguish from chorionepithelioma.
Chorionepnthelioma (chorionic carcinoma). A malignant trophoblast tumor develops from the remains of the placenta. Described
M.N. Nikiforov (1886) and Marchant (1887). Consists of cyto- and syncytiotrophoblast elements. There is no tumor stroma; the vessels look like cavities in which tumor cells float. Hematogenous metastases are characteristic. The tumor is hormonally active and simulates pregnancy. Sometimes ectopic chorionepitheliomas occur: in the mediastinum, testicles in men, bladder, ovary in women.
Tumors are numerous. We will look at the most important ones.
Syringoadenoma. Benign tumor of the epithelium of the sweat gland ducts. Characterized by lining with a two-layer epithelium, the formation of papillae and tubules.
Hidradenoma. A benign tumor of the secretory epithelium of the sweat glands, often forming papillae.
Trichoepithelioma. A benign tumor of the epithelium of the hair follicle, typical cysts filled with horny substance.
Basal cell carcinoma (basal cell carcinoma). The tumor develops from the basal cells of the epidermis; the cells are arranged in strands or nests. The tumor grows radially, destroying adjacent tissue, but does not metastasize. May recur.
Malignant tumors of skin derivatives are represented by cancer of the sweat glands, sebaceous glands and hair follicles. They are rare.
Tumors can develop from the epithelium, stroma, sex cord and germinal tissue, and can be benign or malignant. The most important ovarian tumors are reviewed.
Serous cystadenoma. A benign epithelial tumor that looks like a cyst is usually filled with serous fluid. Sometimes papillary proliferation of the epithelium is possible in cysts.
Mucinous cystadenoma (pseudomucinous cystoma). Benign epithelial tumor. The cysts are lined with prismatic epithelium, and the cavity contains mucus. Sometimes the lining epithelium forms papillae. In case of cyst rupture, implantation of cyst cells along the peritoneum is also possible.
Serous cystadenocarcinoma. A malignant epithelial tumor usually has a papillary structure. Implantation metastases in the peritoneum are characteristic.
Pseudomucinous cystcarcinoma (cancer from a pseudomucinous cyst). A malignant epithelial tumor composed of atypical cells forming solid, glandular and cribriform structures. Necrosis is common.
Tekoma. Benign tumor of the sex cord stroma. The structure may resemble a fibroma. This tumor variant is usually hormonally inactive. If the tumor is built from light, epithelial-like cells, then it is usually hormonally active and produces estrogens.
Malignant thecoma. It is characterized by pronounced polymorphism and cell atypia, resembles sarcoma, and is hormonally inactive.
Granulosa cell tumor (folliculoma). Benign tumor of the sex cord. Grows from granulosa. The cells form trabecular and tubular structures. The tumor is hormonally active and produces estrogens.
Malignant granulosa cell tumor (cancer). It is characterized by high cell polymorphism, rapid growth and metastases.
Dysgerminoma. A malignant tumor, formed from the cells of the male reproductive gland, resembles seminoma; lymphocytes are found in the stroma.
Tumors are very diverse. There are:
1) germ cell tumors;
2) tumors from gonadal stroma cells;
3) tumors arising from the membranes of the testicle and appendages;
4) tumors developing from germinal elements and cells of the gonadal stroma.
Seminoma (dysgerminoma). A malignant tumor built from germinal atypical epithelium. The most common tumor. It metastasizes quite early. Often accompanied by necrosis.
Leydig cell tumor (Leydigoma). Develops from glandulocytes - cells of the gonadal stroma, benign, hormonally active.
Sertoli cell tumor. A benign tumor of sustentocytes, hormonally active, causes premature puberty in children.
Tumors from germ cells and gonadal stroma cells (gonadoblastoma). They develop from seminoma-type cells and cells resembling sustentocytes and granulosa cell elements. Usually the germ cell component metastasizes.
THYROID GLAND. Tumors can arise from cells A, B, C, and can be benign or malignant.
Follicular adenoma. It arises from cells A and B and is similar in structure to the thyroid gland.
Solid adenoma. Develops from C cells, which produce calcitonin. Sometimes it forms papillae. The presence of the latter is an unfavorable sign regarding malignancy.
Thyroid cancer. More often it develops from a previous adenoma.
Follicular cancer. A malignant analogue of follicular adenoma. Constructed from atypical cells. Gives predominantly hematogenous metastases to the lungs and bones.
Papillary cancer. The most common malignant tumor of the thyroid gland. Constructed from atypical cells that form papillae.
Solid (medullary) cancer. Develops from C cells, which produce calcitonin. It is characteristic that in this cancer, amyloid is often detected in the stroma, which is formed by tumor cells belonging to the APUD system. This amyloid is called APUD amyloid.
Undifferentiated cancer. Constructed from atypical polymorphic cells, there are two variants: small- and giant-cell.
PARATHYROID GLANDS.
Adenoma. It usually has a trabecular structure and is hormonally active. Accompanied by hyperparathyroidism, which causes the development of fibrous osteodystrophy.
Cancer of the parathyroid glands. It has no specific features and is rare.
ADRENAL GLANDS.
Tumors arise from the cortex and medulla. They can be benign and malignant.
Benign tumors of the cortex. Clear cell adrenocortical adenoma. It produces aldosterone and causes Conn's syndrome. This adenoma is also called aldosteroma.
Dark cell adreiocortical adenoma. It produces androgens (androsterome), which is why signs of virilism and, less commonly, Cushing's syndrome occur.
Mixed adreiocortical adenoma. Manifested by hypercortisolism (Cushing's syndrome), called corticosteroma.
Glomerular cell adenoma. Manifested by increased production of mineralocorticoids.
Malignant tumor of the adrenal cortex. Adrenocortical cancer. Constructed from atypical polymorphic cells, it gives predominantly hematogenous metastasis.
Benign brain tumor. Pheochromocytoma. This hormonally active tumor releases large amounts of catecholamines, which leads to increased blood pressure.
Malignant tumor of the adrenal medulla. Malignant pheochromocytoma (pheochromoblastoma). Characterized by pronounced cellular atypia, usually hormonally inactive.
THYM GLAND. Tumors arising from cortical and medullary cells are benign and malignant. Clinically, they are asymptomatic or cause myasthenia gravis, immunodeficiency syndromes and autoimmune diseases (systemic lupus erythematosus).
There are 4 types of thymomas.
Cortical cell thymoma. It arises from the cortical epithelium, has infiltrating growth, and has moderate cellular atypia.
Medullary cell thymoma. Originating from the epithelium of the medulla, the tumor is usually benign.
Mixed cell thymoma. Has the symptoms of the previously described thymomas.
Granulomatous thymoma. Constructed from atypical multinucleated epithelial cells that form granulomatous formations.
Adenoma. Histologically, chromophobe, eosinophilic, and basophonic adenomas are distinguished. Typically these tumors have hormonal activity. Depending on the hormonal activity of adenomas, somatotropic and prolactin adenomas are distinguished; adenoma of ACTH-secreting cells; adenoma secreting thyroid-stimulating hormone; an adenoma of cells that secrete follicle stimulating hormone.
Pinealoma. Benign tumor of glandular epithelium and neuroglia; causes metabolic and hormonal disorders.
PANCREAS.
Tumors of the islet apparatus of the pancreas belong to tumors of the APUD system (apudomas). The following tumors are distinguished.
Insulinoma. Develops from B cells of the islet apparatus. Its structure resembles a trabecular or tubular adenoma. Hormonally active, cells produce large amounts of insulin, which leads to the development of hypoglycemic syndrome.
Gastrinoma. Develops from G cells. It can be multiple. Its structure resembles a trabecular adenoma. Hormonally active, the cells produce large amounts of gastrin, which leads to the development of Zollinger-Ellison syndrome.
Glucagonoma. Appears from A-cells that synthesize glucagon. The structure has the appearance of a trabecular adenoma. Causes a hyperglycemic state and the development of diabetes mellitus.
Vipoma. Develops from D 1 cells that produce a hormone similar to vasoactive intestinal polypeptide hormone (VIP). The structure is solid-trabecular, causing hypokalemia and dehydration.
Srotoninoma. It arises from E c cells that produce 5-hydroxytryptamine. It has a solid-trabecular structure and causes carcinoid syndrome.
Somatostatinoma. D-cell adenoma. The structure resembles a solid trabecular adenoma; Hypoinsulinemia, hypoglucagonemia, steatorrhea, and achlorhydria are typical.
All these tumors have malignant analogues, which can be hormonally active; they are called malignant insulinomas, gastrinomas, etc.
GASTROINTESTINAL TRACT.
Carcinoid. It develops in the mucous membrane of the gastrointestinal tract from enterochromaffin cells that produce various biogenic amines (most often serotonin). The structure of the tumor resembles a solid trabecular adenoma and produces an argentaffin and chromaffin reaction. The cells of this tumor belong to the APUD system. In patients they cause carcinoid syndrome.
Malignant carcinoid. Malignant analogue of carcinoid.
Source:
Connective (fibrous) tissue
Adipose tissue Muscle tissue
Blood vessels
Lymphatic vessels
Synovial membranes
Mesothelial tissue Bone tissue
Benign
Fibroma: dense, soft, desmoid
Dermatofibroma (histiocytoma)
Lipoma Hibernoma
Leiomyoma Rhabdomyoma Granular cell tumor
Hemangioma: capillary, venous, cavernous; benign hemangio-pericytoma Glomus tumor (glomus angioma)
Lymphangioma
Benign synovioma
Benign mesothelioma
Osteoma, benign osteoblastoma
Chondroma, benign chondroblastoma Giant cell tumor
Malignant
Fibrosarcoma. differentiated, undifferentiated
Dermatofibroma protuberans (malignant histiocytoma)
Liposarcoma Malignant hibernoma
Leiomyosarcoma Rhabdomyosarcoma Malignant granular cell tumor
Angiosarcoma: malignant hemangioendothelioma, malignant hemangiopericytoma
Lymphangiosarcoma (malignant lymphangioendothelioma)
Synovial sarcoma (malignant synovioma)
Malignant mesothelioma
Osteosarcoma Chondrosarcoma
Benign tumors
Fibroma. A connective tissue tumor is built from cells such as fibroblasts, fibrocytes and bundles of collagen fibers. There are two types of fibromas: dense, with a predominance of bundles of collagen fibers, and soft, built from a large number of cells and loose connective tissue. Desmoid is a special type of fibroma characterized by infiltrating growth. After removal it recurs. Occurs predominantly in women.
Dermatofibroma (histiocytoma). Constructed from cells such as fibroblasts, histiocytes, macrophages, fibrocytes. Characterized by large multinucleated cells containing hemosiderin and lipids (Touton cells). It is most often found on the skin of the extremities.
Lipoma. Constructed from lipocytes of adipose tissue, it can be single or multiple. Sometimes it grows, infiltrating the muscles (intramuscular, or infiltrating, lipoma).
Hibernoma. Develops from brown fat cells, usually solitary. Most often localized in the interscapular region of the back.
Leiomyoma. It arises from smooth muscles, bundles of muscle fibers are chaotically intertwined. To distinguish it from fibroma, the tissue is stained using the Van Gieson method. If the stroma is highly developed, then they speak of fibromyoma.
Rhabdomyoma. Develops from striated muscle cells resembling embryonic muscle fibers.
Granular cell tumor (Abrikosov tumor). It is of neurogenic origin, developing from Schwann nerve sheath cells. Most often localized in the language.
Hemangioma. Collective concept. The following types are distinguished:
Capillary hemangioma - built from branching capillary-type vessels, most often localized in the skin;
Venous hemangioma - built from vessels that form cavities resembling veins;
Cavernous hemangioma - consists of large thin-walled vascular cavities filled with blood (found in the liver, skin);
Benign hemangiopericytoma is built from chaotically intertwined capillaries surrounded by couplings of proliferating pericytes.
Glomus tumor (glomus angioma). Constructed of vessels surrounded by glomus cells, rich in nerve fibers.
Lymphangioma. Constructed of lymphatic vessels of various shapes and sizes filled with lymph.
Benign snovioma. Develops from synoviocytes of tendon sheaths and tendons. Builds alveolar structures. Often forms giant cells. Sometimes xanthoma cells are found in the tumor.
Benign mesothelioma. It arises from the mesothelium and resembles a fibroma.
Osteoma. Constructed of bone beams separated by fibrous tissue, a distinction is made between spongy and compact osteomas.
Benign osteoblastoma. Consists of small osteoid, partially calcified bone beams separated by fibrous tissue with osteoclasts.
Chondroma. It is built from randomly arranged cells of hyaline cartilage (if the tumor is localized in the peripheral parts of the bone, then it is called ecchondroma, if in the central parts - enchondroma).
Benign chondroblastoma. It differs from chondroma only in that chondroblasts are found in it.
Giant cell tumor. Constructed of giant cells and fibrous tissue, it also contains xanthoma cells. The histogenesis of this tumor is unclear; it recurs and sometimes metastasizes hematogenously.
Malignant tumors
Malignant mesenchymal tumors have pronounced cellular atypia and are called sarcoma. Metastasis by hematogenous route is typical.
Fibrosarcoma. Malignant connective tissue tumor. Constructed from atypical fibroblast-like cells. Depending on the degree of differentiation, differentiated fibrosarcoma, which metastasizes late, and poorly differentiated fibrosarcoma, which is characterized by early metastases, are distinguished.
Dermatofibroma protuberans (malignant histiocytoma). It differs from its benign counterpart in the presence of atypical cells with mitoses. It grows slowly and rarely metastasizes.
Liposarcoma (lipoblastic lipoma). Malignant tumor of adipose tissue. There are several types of it: predominantly highly differentiated, predominantly myxoid, predominantly round cell, predominantly polymorphic cellular. Liposarcoma grows slowly and metastasizes late.
Malignant hibernoma. It differs from its benign counterpart by pronounced cellular polymorphism. Sometimes giant cells are found.
Leiomyosarcoma. Malignant smooth muscle tumor. It is characterized by pronounced cell polymorphism and a large number of mitoses.
Rhabdomyosarcoma. Rare malignant tumor of striated muscle. It has a polymorphic structure, which often complicates differential diagnosis.
Malignant granular cell tumor. It differs from its benign counterpart by pronounced cell polymorphism and mitoses.
Aniosarcoma. Malignant tumor of blood vessels. It is characterized by pronounced cellular atypia and originates from endothelial cells (malignant hemangioendothelioma) or pericytes (malignant hemangiopericytoma). The tumor is characterized by rapid growth and early metastases.
Lnmphangiosarcoma. Malignant tumor of the lymphatic vessels.
Synovial sarcoma (malignant synovioma). It has a monophasic or biphasic structure of cells forming pseudoepithelial glandular formations and atypical fibroblast-like cells. It grows quickly and metastasizes early.
Malignant mesothelioma. Develops in the peritoneum and pleura. Constructed of atypical cells forming papillary or tubular structures.
Osteosarcoma (osteogenic sarcoma). Constructed from atypical cells such as osteoblasts with many mitoses and primitive bone. Depending on the characteristics of bone formation, osteoblastic and osteolytic forms are distinguished.
Chondrosarcoma. It is characterized by pronounced polymorphism of atypical cells of the chondroid type, the formation of chondroid intercellular substance. There may be foci of osteogenesis and mucus formation. It grows slowly and gives late metastases.
WHO experts (1969) identified a group of soft tissue tumors. It includes tumors of all nonepithelial extraskeletal tissues, with the exception of the lymphoreticular system, as well as neuroectodermal tumors of the peripheral nervous system and nerve ganglia.
Tumors of melanin-forming tissue
Melanin-forming cells (melanocytes) arise from the Schwann sheath of peripheral nerves. Tumor-like formations are represented by nevi, true tumors are represented by melanoma.
Nevi. Most often found in the skin. Depending on the location there are:
1) border nevus, growing at the border of the epidermis and dermis; intradermal, located only in the dermis; complex (mixed), characterized by the features of borderline and intradermal nevi; epithelioid (spindle cell), found in children (juvenile nevus), multinucleated giant cells are sometimes detected in it.
Melanoma (melanosarcoma, malignant melanoma). One of the most malignant human tumors. It grows quickly and metastasizes very early, both hematogenously and lymphogenously. Localized wherever there are pigment cells. The structure of the tumor is polymorphic, the cells have pronounced cellular atypia. According to Clark's classification, the following forms are distinguished: superficial spreading, lentigo maligna type, nodular. The depth of tumor growth into the dermis and subcutaneous tissue (fatty tissue) is of decisive importance for the prognosis of a tumor - there are 5 stages. Melanoma can be pigmented or non-pigmented.
Tumors of the nervous system and meninges
Tumors can originate from the central, autonomic (autonomic), peripheral nervous system and the mesenchymal elements included in this system (Table 5). Tumors can be benign or malignant. It should be noted that when tumors are localized in the central nervous system, regardless of the structure, the course of all tumors is malignant. Another feature of CNS tumors is that they metastasize within the brain and spinal cord.
Tumors of the central nervous system
Tumors of the central nervous system are divided into neuroectodermal and meningovascular.
Neuroectodermal tumors
Neuroectodermal tumors most often develop from glial elements and are represented by various benign and malignant gliomas.
Glial tumors
Astrocytoma. A benign tumor, one of the most common tumors of a neuroectodermal nature. Develops from astrocytic glial cells. Depending on the structure, fibrillar, protoplasmic, fibrillar-protoplasmic astrocytoma is distinguished.
Astroblastoma. A malignant analogue of astrocytoma. It is characterized by cellular atypia, rapid growth, necrosis and metastases within the central nervous system.
Oligodendroglioma. Benign tumor of oligodendroglia. Sometimes calcifications and cysts are found in it.
Oligodendroglioblastoma. A malignant tumor with pronounced cellular atypia and the presence of foci of necrosis. Growing quickly.
Ependymal and choroidal epithelial tumors
Eiendymoma. A benign tumor of glial nature, developing from the ependyma of the ventricles of the brain; forms pseudorosettes around the vessels.
Ependymoblastoma. Malignant tumor. Characterized by pronounced cellular polymorphism, reminiscent of glioblastoma. It grows quickly and metastasizes within the central nervous system.
Choroid papilloma (choroid papilloma). A benign tumor develops from the epithelium of the choroid plexus of the brain and consists of villous growths of the epithelium.
Choroid carcinoma (malignant choroid papilloma). Located in the ventricles of the brain, built from atypical choroidepithelial cells (papillary cancer). Rarely seen.
Neuronal tumors
Ganglioeuroma (gangliocytoma). A benign tumor composed of mature ganglion cells. Rarely seen.
Gaiglioneroblastoma. A very rare tumor, a malignant analogue of ganglioneuroma.
Neuroblastoma. Built from neuroblasts, includes many mitoses, cells grow, forming syncytium. Rarely seen.
Poorly differentiated and embryonal tumors
Medulloblastoma. A very malignant tumor composed of medulloblasts. It occurs in children and is usually located in the cerebellar vermis.
Glioblastoma. One of the most common malignant brain tumors. It is characterized by pronounced cellular atypia, necrosis and hemorrhage. The tumor grows quickly and metastasizes early.
Meningovascular tumors
Tumors arise from the membranes of the brain and related tissues.
Meningioma (arachnoidendothelioma). A benign tumor emanating from the soft meninges is built from endothelial-like cells, in which lime is often deposited and psammoma bodies are formed. Depending on the structure, there are several types of meningiomas.
Meningeal sarcoma. A malignant analogue of meningiomas. The structure resembles fibrosarcoma.
Tumors of the autonomic nervous system
Tumors of the autonomic (autonomic) nervous system, developing from sympathetic ganglia, as well as cells of non-chromaffin paraganglia, can be benign and malignant.
Benign non-chromaffin paraganglioma (chemodectoma). It develops from cells belonging to the APUD system, capable of synthesizing serotonin, and less commonly ACTH, which is why this tumor is called apudoma. It has a trabecular structure and contains a large number of sinusoidal vessels.
Malignant nonchromaffin paraganglioma. Has pronounced cellular polymorphism. Characterized by infiltrating growth and lymphogenous metastases.
Sympathoblastoma (sympathogonioma). The structure of a malignant tumor resembles neuroblastoma. Cells with pronounced cellular atypia, many mitoses. The tumor grows quickly and metastasizes early. Occurs in childhood.
Tumors of the peripheral nervous system
Tumors of the peripheral nervous system that develop from nerve sheaths can be benign or malignant.
Neurolemmoma (schwannoma). It is built of spindle-shaped cells forming rhythmic structures (palisades) called “Verokai bodies”.
Neurofibroma. Consists of nerve fibers and connective tissue. If the patient has systemic neurofibromatosis, then we are talking about Recklinghausen's disease.
Malignant neurilemmoma (neurogenic sarcoma). It is characterized by pronounced cellular atypia and polymorphism; rhythmic structures and nuclear symplasts can be found in it.
Tumors of the blood system
Teratomas. They develop when one of the blastomeres of the egg breaks off. Consist of one or more types of fabrics. They can have an organismoid and organoid structure. They often reach large sizes. The most common teratomas are sacrococcygeal, ovarian, testicular, retroperitoneal and mesenteric, pharynx, and lung.
Teratoblastoma. A malignant tumor, characterized by pronounced cellular atypia and polymorphism, grows rapidly and metastasizes.
Contents of the article
Cancer of the uterus- hormone-dependent tumor. Currently, there is an increase in the incidence of uterine cancer throughout the world, which is associated with dysfunction of the endocrine system (hyperestrogenism, obesity, diabetes mellitus). A cancerous tumor develops from the epithelium (usually columnar) lining the mucous membrane and glands of the uterine mucosa. In some cases (very rarely), uterine cancer can develop from ectopic stratified squamous epithelium. There is a frequent combination of uterine cancer with menstrual irregularities (especially during menopause), Stein-Leventhal syndrome, uterine fibroids, hormone-producing ovarian tumors, obesity, diabetes mellitus and hypertension.Cancer of the uterus develops, as a rule, against the background of hyperplastic processes in the endometrium, caused by prolonged proliferation of endometrial glands without their transition to the secretory phase. These processes are often caused by hyperestrogenemia. Constant and long-term (more than 10-15 years) exposure to estrogen on endometrial cells, especially when liver function is impaired, leads to their increased mitosis and transformation into an atypical form. It is known that the liver is involved in the metabolism of hormones, which, when destroyed, are removed from the body in the form of metabolites. When liver function is impaired, a significant amount of estrogen accumulates in the body, which affects not only the uterus, but also the pituitary gland, ovaries, adrenal, thyroid and pancreas, causing their hyperfunction. This in turn leads to disruption of protein, carbohydrate and fat metabolism, and often to the development of obesity, diabetes and hypertension.
Currently, the following factors determine the development of uterine cancer are identified:
long-term ovulation disorder, causing hyperestrogenemia, which in turn leads to hyperplasia and proliferation of the endometrium without transition to the secretory phase;
decreased reproductive function and infertility due to menstrual irregularities (hypomenstrual syndrome, amenorrhea associated with anovulation processes);
obesity caused by hyperfunction of the anterior pituitary gland (there is a statistically significant relationship between uterine cancer and obesity);
hypertension, especially in combination with obesity; diabetes mellitus, which is a consequence of carbohydrate metabolism disorders (decreased tolerance to carbohydrates);
liver dysfunction, causing disruption of the metabolism of hormones, especially steroids, which leads to an increase in their activity even with a normal level of secretion;
feminizing ovarian tumors(theca cell and granulosa cell, Brenner tumor), leading to hyperestrogenemia;
Stein-Leventhal syndrome(bilateral polycystic ovarian changes, amenorrhea, infertility, ovulation disorders, hirsutism, obesity);
cell hyperactivity the inner tunic of the follicle during menopause, when they become the main source of estrogen;
follicular ovarian cyst, in which the feedback mechanism between the pituitary gland and the ovaries is disrupted; glandular and, especially, glandular-cystic hyperplasia of the endometrium with a tendency to polyp formation: uterine fibroids, combined with increased production of estrogen; endometriosis of the uterine body with growth into the myometrium.
Histologically, the following forms of uterine cancer are distinguished:
well-differentiated glandular cancer (malignant adenomatosis, malignant adenoma);
mature glandular cancer;
glandular solid cancer;
solid (poorly differentiated) cancer; adenoacanthoma.
Less differentiated forms are more common in old age, more mature ones - in reproductive age and especially in patients with a history of ovulation and menstruation disorders. Well-differentiated glandular cancer develops against the background of hyperplastic processes or endometrial adenomatosis. In its pathogenesis, disruption of ovulation, as well as the metabolism of fats and carbohydrates, is important.
Highly differentiated glandular cancer consists of glandular formations that are located incorrectly, although they retain a tubular structure. Branching glandular elements of various sizes with different lumen shapes. The glandular epithelium is cylindrical, located single-row or multi-row, the nuclei are hyperchromic. Between the glands there is a thin connective tissue layer. Nuclear mitoses are common.
Mature glandular cancer consists of ferruginous conglomerates with intricate labyrinths of glands. Cancer cells destroy their own membrane, causing the glandular cavities to merge with each other. There is a tendency towards infiltrating growth into the thickness of the uterine wall. The glandular epithelium is single-row and multi-row.
The atypicality of the glandular epithelium is sharply expressed: cell polymorphism, nuclear hyperchromatosis, giant mononuclear and multinucleated cells. The glandular epithelium tends to form papillary projections into the lumen of the glands, and many irregular mitoses occur.
Glandular solid cancer is characterized by a “glandular-solid structure. The solid structure predominates, in its areas there are small glandular cavities. Cancer cells lose the character of the glandular epithelium. Their pronounced polymorphism and a large number of mitoses are noted. Solid areas of the cancer tumor have a more pronounced destructive growth than glandular ones, destroying the latter As they grow, they fill the glandular cavities, leaving gaps and gaps between the growths.
Solid (poorly differentiated) cancer is a rare form of uterine cancer and is characterized by complete loss of the glandular structure. There are continuous fields of solid structure made up of small cancer cells with sharply hyperchromatic nuclei. There is no cell polymorphism observed (the tumor is very similar to basal cell carcinoma of the cervix).
Adenoacanthoma is characterized by the inclusion of islands of squamous epithelium in the glandular tissue of the tumor. Rarely seen.
Primary squamous cell carcinoma of the uterine body is very rare; it grows in depth, quickly infiltrates tissues and metastasizes.
Histological diagnosis of uterine cancer is not difficult. Abundant scraping from the uterine cavity aims at the correct diagnosis.
The most common glandular form (of varying maturity) of uterine cancer is adenocarcinoma. The glandular epithelium is arranged in regular rows, often single-row, cylindrical, not secerated, with mitoses and atypical nuclei, destroys the basement membrane, penetrates into the thickness of the uterine wall. In some cases, the cancer loses its glandular structure and penetrates into the underlying tissue in the form of continuous masses, no different in its histological structure from squamous cell carcinoma, in which cells in places can become keratinized and form well-defined “pearls.”
Clinical forms of uterine cancer
In the general structure of malignant neoplasms of the female genital organs, uterine cancer ranks second after cervical cancer.Uterine cancer most often develops after 50 years of age. It has a more favorable clinical course than cervical cancer, spreads more slowly to the underlying tissues and metastasizes late to distant organs. Cancer of the uterine body can spread diffusely, affecting the entire surface of the endometrium, or focally.
According to the nature of growth (regardless of the histological structure), a distinction is made between the exrphytic papillary - warty, polypous and in the form of an isolated large node), which is more common, and the endophytic form. More often the tumor is localized in the area of the fundus or angle of the uterus, less often in the area of the lower segment. The malignant nature of the tumor is manifested in its destructive growth into the myometrium, neighboring organs (bladder, rectum) and metastasis to the lumbar lymph nodes.
Distribution routes
Cancer of the uterine body metastasizes mainly by the lymphatic route, less often by hematogenous route. Lymph from the middle and upper parts of the uterine body is collected in the plexus subovaricus, from where it enters the lower and upper lumbar lymph nodes. Regional metastases of uterine body cancer are more often localized in the lumbar and less often in the inguinal lymph nodes.It has been proven that metastasis of uterine body cancer can occur through the lymphatic tract going to the lymph nodes located in the area of the external and internal iliac arteries, as well as in the area of the obturator foramen (if the cancer process is localized in the lower segment of the uterus - the isthmus).
The initial formations of the uterine lymphatic system are intertissue gaps and capillary networks located in the endometrium, myometrium and perimetry. The efferent lymphatic vessels of the uterus are formed mainly in the external muscular and subserosal lymphatic plexuses, in which there are numerous anastomoses of the lymphatic vessels of the cervix and the body of the uterus. There is no isolation of the lymphatic network of the cervix and the body of the uterus, and therefore, for cancer of the uterine body, an operation is recommended - extended hysterectomy according to the Gubarev-Wertheim method, in which the lymph nodes located along the external and internal iliac arteries are removed in the area obturator foramen, as well as the sacral lymph nodes.
Cancer of the uterine body often (50%) metastasizes by lympho-implantation and implantation (parietal and splanchnic peritoneum, greater omentum) mainly through the fallopian tubes, the ovarian ligaments and the ovary.
Clinical and anatomical classification of skein body cancer
There are the following stages of uterine cancer:Stage I- cancer of the uterine body is limited to the endometrium.
Stage II
a) cancer with myometrial infiltration;
b) cancer with infiltration of the parametrium on one or both sides without transfer to the pelvic wall;
c) cancer with transition to the cervix.
Stage III
a) cancer with infiltration of the parametrium on one or both sides, spreading to the pelvic wall;
b) cancer with metastases to regional lymph nodes, appendages, vagina;
c) cancer with germination of the uterine peritoneum without involvement of neighboring organs in the process.
IV stage
a) cancer with damage to neighboring organs (bladder, rectum);
b) cancer with distant metastases.
Clinical groups of uterine cancer are the same as for cervical cancer.
Currently, they use the international classification of uterine cancer according to the TNM system, where T is the primary tumor, N is regional lymph nodes, M is distant metastases. This classification takes into account the extent of tumor spread.
Uterine Cancer Clinic
As a rule, uterine cancer is characterized by three symptoms: leucorrhoea, bleeding, pain (in late stages).Leucorrhoea appears more often in the form of ichor (pus mixed with blood), then has the character of meat slop, and can be abundant or moderate. Leucorrhoea is usually odorless and does not bother the patient much.
Bleeding can vary in nature - from spotty to heavy. They can be either cyclic (less often) or acyclic (more often). Uterine cancer is characterized by uterine bleeding during menopause. Sometimes anemia may develop, especially when uterine cancer is combined with submucosal fibromatous nodes. Sometimes tissues of disintegrating adenocarcinoma are released from the uterine cavity. Anemia, as a rule, is accompanied by increased ESR, general weakness, malaise, and intoxication.
The pain is usually cramping in nature, which indicates contraction of the walls of the uterus in an effort to expel the contents from the cavity. Pyometra often occurs. Symptoms of compression of adjacent organs (bladder, ureters and rectum) appear in advanced stages of cancer.
It should be remembered that bleeding during menopause is also observed with necrotization of benign polyps, with senile atrophy of the uterine mucosa, atherosclerosis of endometrial vessels (with hypertension), decaying submucosal myoma and endometrial tuberculosis.
The following main stages and forms of the clinical course of uterine cancer are distinguished (Ya.V. Bokhman). Cancer does not develop against the background of a normally functioning endometrium. It is preceded by hyperplastic processes, adenomatosis or atrophy of the mucous membrane.
First stage The clinical course of uterine cancer covers the period from the onset of invasive cancer to deep germination into the myometrium and is characterized by a decrease in the degree of differentiation of endometrial cells.
Second stage- local-regional distribution. The tumor grows deeply into the myometrium, destroys its lymphatic plexuses with the formation of metastases.
Third stage characterized by dissemination of the process from tumor germination beyond the serous membrane to widespread lymphogenous, lymphohematogenous and implantation dissemination.
Identification of three main stages of the clinical course of uterine cancer allows us to justify the sequence of application of preventive and therapeutic measures.
At the first stage, timely diagnosis allows one to achieve good results using both surgical and radiation methods. At the second stage, studying the extent of tumor spread becomes important, which is fundamental for prescribing rational surgical and radiation therapy, and at the third stage - chemotherapy and hormonal therapy.
There are the following forms of clinical course of uterine cancer. Slow, relatively favorable clinical course. The duration of uterine bleeding is due to hyperplastic processes in the endometrium. Histologically, highly differentiated, or mature, glandular cancer with superficial invasion into the myometrium is determined. There are no lymphogenous metastases.
Unfavorable clinical course
The duration of the disease is short. Histologically solid cancer. Acute, extremely unfavorable clinical form of the course. It is rare and is characterized by a combination of a group of unfavorable factors: medium or low differentiation, intense invasive growth, metastases to the pelvic and lumbar lymph nodes.Diagnosis of uterine cancer
The most common and accurate diagnostic method is separate diagnostic curettage of the mucous membrane of the cervical canal and the body of the uterus or vacuum aspiration of the contents of the uterine cavity, followed by histological examination of the scraping. If, during curettage, large fragile masses are removed, preliminary (before the histological conclusion) one can think about uterine cancer. However, if a scant scraping is obtained, uterine cancer cannot be ruled out. In such cases, you should carefully check (with a curette) all the walls of the uterus (especially the fundus and corners).For the purpose of topical diagnosis of uterine cancer, metro- or hysterocervicography is used (the agreement between the topical diagnosis before surgery and the data obtained after surgery is 97%).
Hysterocervicography is one of the leading methods for x-ray diagnosis of cancer of the body and cervical canal.
The X-ray picture of uterine cancer is determined by the size, shape and location of the tumor. To obtain a cervicogram, an additional photograph is taken in the posterior projection immediately after removing the hysterography device from the cervical canal.
Hysterograms with exophytic tumor growth show filling defects in the form of protrusions into the uterine cavity with corroded contours. When the tumor is localized in the area of the tubal angle, the symptom of “horn amputation” is noted. In the endophytic form of cancer, the relief of the mucous membrane is uneven and jagged. In the diffuse form, there is deformation of the uterine cavity with filling defects of a bizarre shape.
When the tumor spreads beyond the isthmus of the uterus, a sharp expansion of the canal and erosion of its contour are noted (with cervicography).
To identify regional metastases and the extent of tumor spread along the lymphatic tract, lympho-, phlebo-, arterio-, pelveo- and urography are used. It goes without saying that all these methods cannot be used to study one patient. Each of the methods listed above has its own indications and contraindications. The most commonly used is lymphography. The principle of the method is that contrast agents (iodolipol or myodil), injected into the lymphatic vessel of the dorsum of the foot and spreading along the lymphatic tract, enter the inguinal and paravertebral lymph nodes. X-ray shows a violation of the shape, structure, size and contours of the affected lymph nodes. On the part of the lymphatic vessels, these signs are expressed in blockade, the development of unusual collaterals, anastomoses and lymphostasis. The leading symptom in the diagnosis of lymphogenous metastases is a marginal defect in the filling of the node with smooth and clear contours.
It is customary to distinguish between a sectoral filling defect (“amputation”) of the pole of the lymph node and a filling defect, in which only a small portion of the crescent-shaped node remains contrasted. The sectoral filling defect corresponds to the initial phase of metastasis, when tumor cells settle in one of the marginal sinuses, most often in the upper pole of the lymph node. As the metastasis grows, it can almost completely displace the lymphoid tissue, leaving only a narrow rim (crescent shadow). The size of the lymph nodes is of relative importance: metastases can be in small nodes and vice versa.
A fairly accurate method of hysteroscopy. However, its utility is limited in clinical practice due to the lack of advanced hysteroscopes. With hysteroscopy, it is possible to directly monitor the condition of the uterine mucosa.
In gynecological practice, the method of cytological diagnosis of uterine cancer is widely used. However, it is an auxiliary method.
Recently, a radioisotope method has been used to diagnose uterine cancer. After ingesting radioactive phosphorus (P32), which has a relatively short half-life and to which endometrial cells are sensitive (the target organ for phosphorus is the cells of the endometrial glands), radiometry of the uterine cavity is performed on the second day using a miniature uterine probe-counter. With endometrial cancer, focal radioactivity increases sharply. However, the radioisotope method for diagnosing uterine cancer using a radiometer, as well as the scanning method (gamma topography), have not found widespread use in diagnosing uterine cancer due to a significant percentage of diagnostic errors. The final method for diagnosing uterine cancer is a biopsy of a scraping obtained from the uterine cavity during diagnostic curettage of its mucous membrane.
Treatment of uterine cancer
For uterine cancer, three main treatment methods are used: combined (surgical method followed by remote X-ray or radiotherapy), combined (including remote X-ray or gamma therapy in combination with intracavitary application of radioactive substances) and complex treatment method, including surgery, radiation and hormone treatment. In advanced cases of the disease, palliative treatment methods are used along with hormonal and chemotherapy.Combined method.
1. Surgical treatment. When cancer is localized in the area of the body and fundus of the uterus, extirpation of the uterus with appendages is indicated; in the area of the lower segment or cervix - extended extirpation of the uterus according to the Gubarev-Wertheim method, i.e. extirpation of the uterus with appendages with additional excision in a single block of the common and external iliac, obturator and internal iliac lymph nodes along with parametrial, pararectal, paravesical, paravaginal and paraobturator (located in the area of the obturator foramen) tissue.2. External beam radiotherapy. In most specialized institutions (gynecological oncology), after surgery (from the 9-10th day), remote X-ray or gamma therapy is prescribed. The total dose of external radiation reaches 12,000-16,000 R, which corresponds approximately to the dose at point A on average 1500-1800 rad, at point B -3000-4000 rad.
Combined method.
1. Remote X-ray or gamma therapy It is carried out, as a rule, from four fields at 200-250 R per session from two fields daily. The total dose of external radiation is the same as in the postoperative period.2. Intracavitary application of radioactive substances- introduction into the uterine cavity of radioactive cobalt needles, cylindrical or round-shaped preparations in the form of beads (there are linear, chain, T-shaped and U-shaped methods for filling the uterine cavity). With intracavitary (intrauterine) treatment, the dose to point A reaches 7000-9000 rad, to point B 2000-2400 rad. Sessions of intracavitary curitherapy are carried out once a week and last 48 hours, during which the focal dose is 1500-2000 rad. For 4-5 applications, patients receive from 8,000 to 10,000 rads.
Hormone therapy
Widely used (especially in advanced stages) and for relapses of 17-a-hydroxyprogesterone capronate (17-a-HPC) or methoxyprogesterone acetate. The effect of 17-HPA on the endometrium and myometrium is very similar to the effect of progesterone, but it is two times stronger and four times longer lasting. 250 mg of the drug is administered intramuscularly daily for 4 weeks. The course dose is 7 g. If the drug is effective (reduction of palpable nodes, cessation of tumor growth, reduction or disappearance of metastases, as well as individual symptoms of the disease), continue administration at 250 mg every other day for 4 months, then 250-500 mg per week for several years, sometimes until the end of life. The drug is also administered intrauterinely in the preoperative period, 500 mg daily for a week.The totality of experimental and clinical observations indicates that 17-GPA causes a clearly defined progestational effect, similar to the effect of progesterone under physiological conditions.
Under the influence of 17-GPC, the following changes occur:
reduction in the proliferative activity of cancer cells;
increasing morphological and functional differentiation of cells;
secretory depletion;
atrophic-degenerative changes ending in necrosis and rejection of the tumor or its individual sections.
Methoxyprogesterone acetate is used for advanced endometrial cancer. Administer 250 mg 2 times a week for three months, then 250-500 mg per week for several years.
Cancer remission with this treatment is observed in 40-50% of patients.
Due to the successful use of progestogens in uterine cancer, the use of androgens has now significantly decreased. However, the possibility of prescribing long-term androgens testenate (1 ml of 12% solution) once a week or Sustanon-250 (1 ml once a month) intramuscularly (intermittent courses) for a long time cannot be ruled out.
In preparation for surgery, as well as in the postoperative period (before the start of radiation therapy), chemotherapy drugs (benzotef, thioTEF, cyclophosphamide) are prescribed. In addition, benzotef 48 mg or thioTEF 20 mg is injected into the abdominal cavity during surgery, and immediately after surgery 24 mg benzotef or 10 mg thioTEF is administered intravenously. This administration of chemotherapy is aimed at a cytostatic effect when cancer cells enter the blood and abdominal cavity during surgery.
Considering that the cancer process is considered as a systemic disease of the whole organism, therapy (in addition to basic treatment methods) should include means aimed at increasing the body’s reactivity (ACS, zymosan, transfusion of blood and its components, splenin, vitamin therapy, hemostimulants, etc. ).
Prevention of uterine cancer
Timely detection and treatment of precancerous conditions (glandular hyperplasia of the endometrium; adenomatous polyps; menstrual irregularities - anovulatory uterine bleeding during menopause); regular gynecological examinations and periodic cytological examinations of smears obtained by aspiration of contents from the vaginal cavity, and, if necessary, from the uterine cavity; special gynecological examinations of women with metabolic disorders (obesity, diabetes, liver disease), accompanied by hyperestrogenism; identification and treatment of patients with feminizing ovarian tumors leading to hyperplastic processes in the endometrium reduces the incidence of endometrial cancer.Malignant tumors that develop from poorly differentiated or undifferentiated epithelial cells are referred to as cancer. The tumor usually has the appearance of a node of soft or dense consistency, its boundaries are unclear, sometimes merging with the surrounding tissue. A cloudy liquid, cancerous juice, is scraped off the whitish surface of the tumor incision. Cancer of the mucous membranes and skin ulcerates early. The following microscopic forms of cancer are distinguished: “cancer in situ” (carcinoma in situ); squamous cell (epidermal) with and without keratinization; adenocarcinoma (glandular); mucous (colloid); solid (trabecular); small cell; fibrous (scirrh); medullary (adenogenic).
"Cancer is in place", or carcinoma in situ (intraepithelial, non-invasive carcinoma) is a form of cancer without invasive (infiltrating) growth, but with pronounced atypia and proliferation of epithelial cells with atypical mitoses. This form of cancer should be differentiated from severe dysplasia. Tumor growth occurs within the epithelial layer, without moving into the underlying tissue. But non-invasive cancer is only a stage of tumor growth; over time, it becomes infiltrating (invasive).
Squamous cell (epidermal) carcinoma develops in the skin and mucous membranes covered with flat or transitional epithelium (oral cavity, esophagus, cervix, vagina, etc.). In mucous membranes covered with prismatic epithelium, squamous cell carcinoma develops only after previous epithelial metaplasia. The tumor consists of strands of atypical epithelial cells that grow into the underlying tissue, destroy it and form nested clusters in it. Tumor cells can retain the ability to keratinize, and then formations resembling pearls (cancer pearls) appear. With a lower degree of cell differentiation, cancer keratinization does not occur. In this regard, squamous cell carcinoma can be keratinizing and non-keratinizing
Adenocarcinoma (glandular cancer) develops from the prismatic epithelium of the mucous membranes and the epithelium of the glands. Therefore, it is found both in the mucous membranes and in the glandular organs. This adenogenic tumor has a structure similar to an adenoma, but unlike an adenoma, in adenocarcinoma there is atypicality of the epithelial cells: they are of different shapes, the nuclei are hyperchromic. Tumor cells form glandular formations of various shapes and sizes, which grow into the surrounding tissue, destroy it, and their basement membrane is lost. There are variants of adenocarcinoma: acinar - with a predominance of acinar structures in the tumor; tubular - with a predominance of tubular formations; papillary, represented by atypical papillary growths. Adenocarcinoma can have varying degrees of differentiation.
Mucous (colloid) cancer is an adenogenic carcinoma, the cells of which have signs of both morphological and functional atypia (perverted mucus formation). Cancer cells produce huge amounts of mucus and die in it.
The tumor has the appearance of a mucous or colloidal mass in which atypical cells are found. Mucous (colloid) cancer is one of the forms of undifferentiated cancer.
Solid cancer(from Latin solidus - single, dense) - a form of undifferentiated cancer with pronounced atypia. Cancer cells are located in the form of trabeculae (trabecular cancer), separated by layers of connective tissue. Mitoses are quite frequent in tumor cells. Solid cancer grows quickly and metastasizes early.
Small cell cancer- a form of undifferentiated cancer, which consists of monomorphic lymphocyte-like cells that do not form any structures; the stroma is extremely scanty. There are many mitoses in the tumor, and necrotic changes are often observed. Growth is rapid, metastases occur early. In some cases, it is not possible to establish the histogenesis of the tumor, then they speak of unclassified cancer.
Fibrous cancer, or scirrhus (from the Greek scirros - dense), is a form of undifferentiated cancer, represented by extremely atypical hyperchromic cells located among layers and strands of coarse fibrous connective tissue. The main feature of this form of cancer is the clear predominance of stroma over parenchyma. The tumor is highly malignant and early metastases often occur.
Medullary(adenogenic) cancer - a form of undifferentiated cancer; its main feature is the predominance of parenchyma over stroma, of which there is very little. The tumor is soft, white-pink in color, and resembles brain tissue (cerebral cancer). It is represented by layers of atypical epithelial cells and contains many mitoses; grows quickly and undergoes necrosis early; gives early and multiple metastases. In addition to those described, there are mixed forms of cancer, consisting of the rudiments of two types of epithelium (flat and cylindrical); they are called dimorphic cancers.
Solid cancer is one of the most aggressive types of epithelial cancer. The name of the disease comes from Latin from solidum, which means “solid”. When viewed under a microscope, such cancer is a group of cells arranged in plates with layers of connective tissue between them.
What organs are characterized by solid cancer?
This cancer can affect the lungs, liver, kidneys, thyroid gland, mammary glands, and other parts of the body and can occur anywhere there is epithelium. Sometimes within the boundaries of the same malignant formation at the same time there are solid fields and other elements.
Solid breast cancer: concept and statistics
This is the most aggressive type of breast cancer. Solid (medullary) breast cancer consists of clusters of abnormal cells. An important feature of such cancer is that it contains undifferentiated cells. They change so much that they become different from ordinary ones. Their almost only goal is regular reproduction. Thus, with a microscope, it is easy to notice many dividing cells in tissues. Solid cancer quickly grows and soon metastasizes. They often become the only and natural way out of the current situation.
Specifics of treatment for solid breast cancer in Israel
In order for the treatment to be as effective as possible, it is very important to identify the disease no later than the second stage, when it is much easier to treat than at the 3rd or 4th stage. An annual examination, together with widespread training of women in the features of self-examination, helps to avoid too late detection of cancer, which also has a positive effect. The neoplasm cannot be left unnoticed, because it is quite painful and mobile. Signs of solid breast cancer also include pink discharge from the nipple and changes in the skin. It is important to note that these are late symptoms; their appearance indicates an advanced stage of the problem.
Today, the treatment of solid cancer is actively carried out in some developed countries, but if this is the USA or European countries, the price may be too high - it is not suitable for all patients. In Israel, the cost of medical services is more affordable, and the level of training of doctors and service is one of the highest in the world. It is known that people even come here from the USA. The latest technologies are being actively introduced in Israel, and the government is investing significant amounts of money in research.
Types of microscopic cancer and why you need to understand what type the formation is classified as?
Solid cancer is one of 8 types of malignant epithelial formations. There are others:

To determine the correct treatment, the doctor must know whether the pathology has grown into nearby organs or spread to the lymph nodes. Using modern technologies, it is possible to create a “molecular portrait” of education.
General information
Tumor, neoplasm, blastoma(from Greek blasto- sprout) - a pathological process characterized by uncontrolled proliferation (growth) of cells; in this case, disturbances in cell growth and differentiation are caused by changes in their genetic apparatus. Autonomous, or uncontrolled growth- the first main property of a tumor. Tumor cells acquire special properties that distinguish them from normal cells. cell atypia, which concerns its structure, metabolism, function, antigenic structure, reproduction and differentiation, is the second main property of a tumor. The acquisition by a tumor cell of new properties not inherent in a normal cell is called anaplasia (from Greek ana- a prefix denoting the opposite action, and plasis- education) or cataplasia (from Greek kata- a prefix denoting movement from top to bottom, and plasis- education).
The terms “anaplasia” and “cataplasia” are ambiguous. Anaplasia is understood as the dedifferentiation of cells, their acquisition of embryonic properties; In recent years, this concept has been criticized, since a fairly high ultrastructural organization of tumor cells and their ability to specifically differentiate have been established. The term “cataplasia” reflects the acquisition of only special properties by a tumor cell; it is more accepted in modern literature.
A tumor can occur in any tissue, any organ, and is observed both in humans and in many animals and plants.
Data epidemiology Oncological diseases indicate different rates of morbidity and mortality from malignant tumors in different countries. The dependence of the occurrence of tumors on natural, biological factors, conditions of the social environment, lifestyle, and everyday habits of certain population groups is shown. According to WHO, up to 90% of tumors are associated with exposure to external factors.
According to statistics, The number of cancer patients and deaths from it is growing in all countries of the world. This is explained both by the deterioration of human ecology and by improved diagnosis of oncological diseases, an established system for registering patients with malignant neoplasms, and a relative increase in the population of elderly and senile people.
Each year, the number of new cases of cancer registered in the world is about 5.9 million. The intensive mortality rate from malignant neoplasms in developed countries is 182 per 100,000, in developing countries - 65 per 100,000. The number of deaths in the world annually from stomach cancer is 575,000, from lung cancer - 600,000, from breast cancer - 250,000. Morbidity and mortality rates from tumors in the world vary greatly. The highest cancer incidence - from 242.3 to 361.1 per 100,000 - was registered in a number of regions of Italy, France, Denmark, the USA, and Brazil.
In Europe, lung cancer and stomach cancer lead in morbidity and mortality. In the United States, in the structure of morbidity in men, the first places are occupied by cancer of the lung, prostate, colon and rectum, in women - breast cancer, cancer of the colon and rectum, and uterine tumors. In Asia and Africa, a large proportion of tumors are malignant lymphoma, hepatocellular and nasopharyngeal cancer.
In the USSR, the absolute number of patients with malignant tumors in 1986 was 641,000 (191.0 per 100,000 population). Of the 544,200 cases, 18% had stomach cancer, 14.3% had lung cancer, 11.3% had skin cancer, and 7.4% had breast cancer. Of the 371,200 deaths, 23.7% were patients with stomach cancer, 18.5% with lung cancer, 5.4% with breast cancer.
Researches tumors oncology (from Greek oncos- tumor). Pathological anatomy solves both theoretical and practical (diagnostic) problems: it describes the structure of tumors, studies the causes of their occurrence, histogenesis and morphogenesis, determines the systematics (classification) of tumors, deals with their intravital and postmortem diagnosis, and establishes the degree of malignancy. For these purposes, all modern methods of histology and cytology are used (Fig. 93).
Rice. 93. Atypical cells, punctate cancer tumor
Structure of the tumor, features of the tumor cell
Appearance tumors are varied. It may be shaped like a knot, a mushroom cap, or resemble a cauliflower. Its surface can be smooth, tuberous or papillary. The tumor may be located in
 Rice. 94. Diffuse growth of a malignant tumor (cancer) in the wall of the stomach
Rice. 94. Diffuse growth of a malignant tumor (cancer) in the wall of the stomach
thicker than an organ or on its surface. In some cases, it diffusely permeates the organ (Fig. 94) and then its boundaries are not defined, in others it is located on the surface of the organ (mucous membrane) in the form of a polyp (Fig. 95). In compact organs, a tumor can protrude above the surface, germinate and destroy the capsule, arroze (corrode) blood vessels, resulting in internal bleeding. It often undergoes necrosis and ulceration (cancer ulcer). On a section, the tumor looks like a homogeneous, usually white-gray or gray-pink tissue, sometimes resembling fish meat. Sometimes the tumor tissue is variegated due to the presence of hemorrhages and foci of necrosis; the tumor may also have a fibrous structure. In some organs (for example, in the ovaries) the tumor has a cystic structure.
Dimensions tumors are different, depending on the speed and duration of its growth, origin and location; consistency depends on the predominance of parenchyma or stroma in the tumor: in the first case it is soft, in the second it is dense.
Secondary changes in tumors they are represented by foci of necrosis and hemorrhage, inflammation, mucus and lime deposits (petrification). Sometimes these changes occur due to the use of radiation therapy and chemotherapy.
Microscopic structure tumors are very diverse. However, all tumors have some common structural features: the tumor consists of parenchyma and stroma, the ratios of which can vary greatly.
Parenchyma tumors form cells that characterize this type of tumor; they determine its morphological specificity. Stroma A tumor is formed both by the connective tissue of the organ in which it developed and by the cells of the tumor itself.
 Rice. 95. Tumor on a stalk in the form of a polyp
Rice. 95. Tumor on a stalk in the form of a polyp
There are complex connections between the parenchyma and tumor stroma, and the characteristics of the tumor parenchyma largely determine the nature of its stroma. As tumor cells grow, they induce the proliferation of fibroblasts and their synthesis of stromal components. This ability of tumor cells is largely determined by their genetic properties; it is unequally expressed in tumors of different histological structures, which explains the different number of fibrous structures in the stroma of different tumors. Tumor parenchyma cells not only induce fibroblast activity, but can themselves produce stromal intercellular substance, or extracellular matrix (for example, collagen type IV basement membranes). Tumor cells, in addition, produce a specific protein substance - angiogenin, under the influence of which capillaries are formed in the tumor stroma.
Most tumors resemble an organ in structure, i.e. have parenchyma and stroma expressed to varying degrees. Such tumors are called organoid. In some, especially undifferentiated, tumors, parenchyma predominates; the stroma is poorly developed and consists only of thin-walled vessels and capillaries. Such tumors are called histioid. They usually grow quickly and undergo necrosis early. In some cases, the tumor is dominated by stroma, with very few parenchyma cells. An example would be fibrous cancer, or skirr.
Tumors whose structure corresponds to the structure of the organ (tissue) in which they develop are called homologous. When the cellular structure of tumors differs from the structure of the organ (tissue) in which they arise, we speak of heterologous tumors. Homologous tumors - mature, differentiated, heterologous - immature, poorly or undifferentiated. Tumors arising from heterotopias, i.e. embryonic displacements are called heterotopic(for example, a bone tumor in the wall of the uterus or lung).
Morphological atypia tumors can be tissue or cellular.
Tissue atypia characterized by a violation of tissue relationships characteristic of a given organ. We are talking about a violation of the shape and size of epithelial structures, the relationship between parenchyma and stroma in epithelial (especially glandular) tumors; about the different thickness of fibrous (connective tissue, smooth muscle, etc.) structures, about their chaotic location in tumors of mesenchymal origin. Tissue atypia is most typical for mature, benign tumors.
Cellular atypia at the light-optical level it is expressed in polymorphism or, on the contrary, monomorphism of cells, nuclei and nucleoli, nuclear hyperchromia (Fig. 96), polyploidy, changes in the nuclear cytoplasmic index in favor of nuclei due to their enlargement, and the appearance of many mitoses.
 Rice. 96. Cellular atypia and tumor polymorphism
Rice. 96. Cellular atypia and tumor polymorphism
Cellular atypia can be expressed to varying degrees. Sometimes it is so significant that tumor cells in appearance become different from the cells of the original tissue or organ. When morphological catplasia reaches an extreme degree, the structure of the tumor is simplified and it becomes monomorphic. In this regard, anaplastic tumors of various organs are very similar to each other.
An important manifestation of morphological atypia of a tumor cell is pathology of mitosis. It has been established that the production of kelons, which under normal conditions regulate the mitotic activity of cells and act as inhibitors of cell division, is impaired in tumor cells. The pathology of mitosis in tumor cells confirms the influence of oncogenic factors on the genetic apparatus of the cell, which determines the unregulated growth of the tumor.
Cellular atypia is characteristic of immature, malignant tumors.
Atypia of ultrastructures, detected during electron microscopic examination, is expressed in an increase in the number of ribosomes associated not only with the membranes of the endoplasmic reticulum, but also lying freely in the form of rosettes and chains, in changes in the shape, size and location of mitochondria (Fig. 97), and the appearance of abnormal mitochondria. The functional heterogeneity of mitochondria is largely mitigated by mitochondria with low or negative cytochrome oxidase activity. The cytoplasm is scant, the nucleus is large with a diffuse or marginal arrangement of chromatin. Numerous membrane contacts of the nucleus, mitochondria and endoplasmic reticulum are revealed, which are extremely marked in a normal cell.
 Rice. 97. Ultrastructural atypia of a tumor cell. M - mitochondria, I - nucleus. x30,000
Rice. 97. Ultrastructural atypia of a tumor cell. M - mitochondria, I - nucleus. x30,000
rarely. Hybrid cells are also an expression of cell atypia at the ultrastructural level (Fig. 98). Atypical undifferentiated cells may include stem cells, semi-stem cells, and progenitor cells.
Electron microscopic examination reveals not only ultrastructural atypia, but also specific differentiation of tumor cells, which can be expressed to varying degrees - high, moderate and low.
 Rice. 98. Hybrid cell (lung cancer). There are signs of an endocrine cell (secretory granules - SG) and type II pneumocyte (osmiophilic multilamellar bodies - MLT). I am the core. x12 500
Rice. 98. Hybrid cell (lung cancer). There are signs of an endocrine cell (secretory granules - SG) and type II pneumocyte (osmiophilic multilamellar bodies - MLT). I am the core. x12 500
At high degree differentiation, several differentiated types of tumor cells are found in the tumor (for example, in a lung cancer tumor, pneumocytes of types I and II, ciliated or mucous cells). At moderate degree differentiation is revealed by one of the types of tumor cells or hybrid cells (for example, in a lung cancer tumor there are only pneumocytes or only mucous cells, sometimes hybrid cells that have ultrastructural characteristics of both a pneumocyte and a mucous cell - see Fig. 98). At low degree differentiation in the tumor, single ultrastructural signs of differentiation are found in a few cells.
The group of differentiated tumor cells detected during electron microscopic examination is also heterogeneous in terms of the severity of specific ultrastructural signs - signs of differentiation: some tumor cells are no different from normal elements of the same type, others have only some specific signs that allow us to talk about being tumor cells. cells to a specific type.
Establishing the degree of differentiation of a tumor cell by electron microscopic examination is important for the differential diagnosis of tumors. Ultrastructural analysis of tumor cells indicates that in an immature tumor with a high degree of malignancy, undifferentiated cells such as stem, semi-stem and progenitor cells predominate. An increase in the content of differentiated cells in a tumor, as well as the degree of their differentiation, indicates an increase in the maturity of the tumor and a decrease in the degree of its malignancy.
Biochemical atypia tumor tissue is expressed by a number of metabolic features that distinguish them from normal ones. It was found out (Shapot V.S., 1977) that the spectrum of biochemical characteristics of each tumor is unique and includes different combinations of deviations from the norm. Such variability of a malignant tumor is natural.
Tumor tissue is rich in cholesterol, glycogen and nucleic acids. In tumor tissue, glycolytic processes predominate over oxidative ones; there are few aerobic enzyme systems, i.e. cytochrome oxides, catalases. Severe glycolysis is accompanied by the accumulation of lactic acid in tissues. This peculiarity of tumor metabolism enhances its similarity with embryonic tissue, in which the phenomena of anaerobic glycolysis also predominate.
Issues of biochemical tumor anaplasia are covered in more detail in the course of pathological physiology.
Histochemical atypia(Kraevsky N.A., Raikhlin N.T., 1967) reflects to a certain extent the biochemical characteristics of the tumor. It is characterized by changes in the metabolism of proteins and, in particular, their functional groups (sulfhydryl and disulfide) in the tumor cell, accumulation of nucleoproteins, glycogen, lipids, glycosaminoglycans and changes in redox processes. In the cells of different tumors, a heterogeneous picture of histochemical
changes, and each tumor is unique histochemically, as well as biochemically. For a number of tumors, specific enzymes (enzyme markers) have been identified; "enzyme profile" characteristic of this type of tumor.
Thus, high activity of acid phosphatase, esterase and nonspecific X-exonuclease, enzymes characteristic of the epithelium of this organ normally, was found in prostate cancer cells. In hepatocellular cancer, unlike cholangiocellular cancer, aminopeptidase is detected; in tumors from the exocrine part of the pancreas, unlike tumors from its islets, high esterase activity remains. A quantitative histochemical study showed that forms of lung, stomach and breast cancer that are histologically and clearly differentiated differ from each other in the activity of a number of enzymes (oxidoreductases).
Antigenic atypism tumor is manifested in the fact that it contains a number of antigens unique to it. Among tumor antigens distinguish (Abelev G.I., 1974): antigens of viral tumors; antigens of tumors caused by carcinogens; transplantation-type isoantigens; embryonic antigens; heteroorgan antigens.
Antigens of viral tumors determined by the viral genome of DNA- and RNA-containing viruses, but belong to the tumor cell. These are nuclear membrane antigens that are identical to any tumors caused by this virus. Antigens for tumors caused by carcinogens are individual both in relation to the carriers of the tumor and its nature. Transplant-type isoantigens found in tumors induced by oncornaviruses (leukemia, breast cancer, etc.). Fetal antigens- tumor antigens specific to the embryonic stages of development of the body and absent in the postnatal period. These include: a 1 -fetoprotein, found most often in cells of hepatocellular carcinoma and embryonal testicular cancer; a 2 -fetoprotein, detected in children with neuroblastoma and malignant lymphoma; carcinoembryonic antigen, which is found in intestinal or pancreatic cancer. Embryonic antigens are detected not only in the tumor, but also in the blood of patients. Heteroorgan antigens- organ-specific antigens that do not correspond to the organ in which the tumor develops (for example, the appearance of a specific kidney antigen in liver carcinoma or, conversely, a liver antigen in kidney carcinoma). In addition to atypical antigens, tumor cells also contain typical species-specific, organ-specific, isoantigens and other antigens.
In undifferentiated malignant tumors occurs antigenic simplification, which, like the appearance of embryonic antigens, is a reflection of cataplasia of the tumor cell. Detection of typical and atypical antigens in a tumor using immunohistochemical methods (including the use of monoclonal antibodies) serves for differential diagnosis and establishment of tumor histogenesis.
Functional properties tumor cells, reflecting tissue and organ specificity, depend on the degree of morphological and biochemical (histochemical) catplasia. More differentiated
tumors retain the functional characteristics of the cells of the original tissue. For example, tumors arising from pancreatic islet cells secrete insulin; tumors of the adrenal glands and the anterior lobe of the pituitary gland secrete a large amount of corresponding hormones and give characteristic clinical syndromes that make it possible to suggest a tumor lesion of these endocrine glands. Tumors from liver cells secrete bilirubin and are often colored green. Poorly differentiated and undifferentiated tumor cells may lose the ability to perform the function of the original tissue (organ), while mucus formation sometimes persists in severely anaplastic cancer cells (for example, stomach).
In conclusion, we can highlight the main phenotypic characteristics of a malignant tumor cell: the tumor cell is more or less aggressive (infiltrating growth), non-communicative (loss of intercellular contacts, cell release from complexes, etc.), but completely non-autonomous. It can reach varying, even high, degrees of differentiation, functioning with different, sometimes minimal, deviations from the norm.
Tumor growth
Depending on degree of differentiation There are three types of tumor growth: expansive, appositional, and infiltrating (invasive).
At expansive growth the tumor grows “out of itself”, pushing away the surrounding tissue. The parenchymal elements of the tissue surrounding the tumor atrophy, stromal collapse develops and the tumor is surrounded, as it were, by a capsule (pseudocapsule). Expansive tumor growth is slow and is characteristic of mature, benign tumors. However, some malignant tumors (kidney cancer, thyroid cancer, fibrosarcoma, etc.) can grow expansively.
Appositional growth tumor occurs due to neoplastic transformation of normal cells into tumor cells, which is observed in the tumor field (see. Morphogenesis of tumors).
At infiltrating (invasive) growth tumor cells grow into surrounding tissues and destroy them (destructive growth). Invasion usually occurs in the direction of least resistance along interstitial gaps, along nerve fibers, blood and lymphatic vessels. Complexes of tumor cells destroy the walls of blood vessels, penetrate the blood and lymph flow, and grow into loose connective tissue. If along the path of tumor invasion there is an organ capsule, membrane and other dense tissues, then the tumor cells first spread along their surface, and then, growing through the capsule and membranes, penetrate deep into the organ (Fig. 99). The boundaries of the tumor during its infiltrating growth are not clearly defined. Infiltrating tumor growth is rapid and is characteristic of immature, malignant tumors.
 Rice. 99. Schematic representation of infiltrating (invasive) growth of a cancer tumor:
Rice. 99. Schematic representation of infiltrating (invasive) growth of a cancer tumor:
1 - atypism and cell polymorphism; 2 - infiltrating growth; 3 - germination of underlying tissues; 4 - atypical mitoses; 5 - ingrowth into lymphatic vessels - lymphogenous metastases; 6 - ingrowth into blood vessels - hematogenous metastases; 7 - perifocal inflammation
In relation to the lumen of a hollow organ tumor growth can be endophytic or exophytic. Endophytic growth- infiltrating tumor growth deep into the organ wall. In this case, the tumor from the surface of the mucous membrane (for example, stomach, bladder, bronchus, intestines) may be almost invisible; A section of the wall shows that it has grown with a tumor. Exophytic growth- expansive growth of a tumor into an organ cavity (for example, stomach, bladder, bronchus, intestines). The tumor can fill a significant part of the cavity, connecting to the wall with its stem.
Depending on number of foci of occurrence tumors talk about unicentric(one outbreak) and multicentric(multiple lesions) growth.
Benign and malignant tumors
Depending on the clinical and morphological characteristics of the behavior, tumors are divided into: 1) benign; 2) malignant; 3) tumors with locally destructive growth.
Benign, or mature, tumors consist of cells that are so differentiated that it is almost always possible to determine from what tissue they grow (homologous tumors). The tumor is characterized by tissue atypia, expansive and slow growth. The tumor usually does not have a general effect on the body and, as a rule, does not metastasize. Due to
Due to the localization feature (brain and spinal cord), benign tumors can sometimes turn out to be dangerous. Benign tumors can become malignant (from lat. malignum- malignant), i.e. become malignant.
Malignant, or immature, tumors consist of poorly or undifferentiated cells; they lose their resemblance to the tissue (organ) from which they originate (heterologous tumors). Characterized by cellular atypia, infiltrating and rapid tumor growth. There are differentiated (highly, moderately and poorly differentiated) - less malignant and undifferentiated - more malignant tumors. Establishing the degree of differentiation, and therefore the degree of malignancy of the tumor, is of great importance prognostic meaning.
Malignant tumors metastasize, recur, and have not only a local, but also a general effect on the body.
Metastasis manifests itself in the fact that tumor cells enter the blood and lymphatic vessels, form tumor emboli, are carried away by the blood and lymph flow from the main node, are retained in the capillaries of organs or in the lymph nodes and multiply there. This is how they arise metastases, or secondary (daughter) tumor nodes, in the liver, lungs, brain, lymph nodes and other organs. The formation of metastases cannot be reduced only to mechanical blockage of capillaries by tumor emboli. In their development, the characteristics of tumor cells are important, expressed in the presence of “highly metastatic” cell phenotypes and “non-metastatic cell” phenotypes in the same tumor. To “select” an organ during metastasis, tumor cells use a receptor system, with the help of which they recognize the “organ-specific affinity” of the blood or lymphatic bed during circulation.
Metastases can be hematogenous, lymphogenous, implantation and mixed. Some malignant tumors (for example, sarcomas) are characterized by hematogenous metastases, for others (for example, cancer) - lymphogenous. About implantation (contact) metastases they say when cells spread along the serous membranes adjacent to the tumor node.
More often, in metastases, the tumor has the same structure as in the main node. Metastasis cells can produce the same secretions and hormones as the cells of the main tumor node. However, tumor cells in metastases may become more mature or, conversely, acquire a greater degree of cataplasia compared to the primary tumor node. In such cases, it is very difficult to determine the nature and localization of the primary tumor node based on the histological structure of the metastasis. In metastases, secondary changes often occur (necrosis, hemorrhage, etc.). Metastatic nodes, as a rule, grow faster than the main tumor node, and therefore are often larger than it.
The time required for metastasis to develop may vary. In some cases, metastases appear very quickly, following the onset
in the absence of the primary node, in others they develop several years after its occurrence. So-called late latent, or dormant, metastases are possible, which occur many (7-10) years after radical removal of the primary tumor node. This kind of metastasis is especially characteristic of breast cancer.
Tumor recurrence - its appearance in the same place after surgical removal or radiation treatment. A tumor develops from individual tumor cells remaining in the tumor field. Tumor relapses can also occur from nearby lymphogenous metastases that were not removed during surgery.
Influence Tumors on the body can be local or general. Local influence The tumor depends on its nature: a benign tumor only compresses the surrounding tissues and neighboring organs, a malignant one destroys them, leading to serious consequences. General influence on the body is especially typical for malignant tumors. It is expressed in metabolic disorders, the development of cachexia (cancer cachexia).
Tumors with locally destructive growth occupy an intermediate position between benign and malignant: they have signs of infiltrating growth, but do not metastasize.
Morphogenesis of tumors
Morphogenesis of tumors can be divided into the stage of pretumor changes and the stage of tumor formation and growth.
Pretumor changes in the vast majority of cases, precede the development of a tumor, but the possibility of developing a malignant tumor is also possible de novo,“right off the bat”, without previous pre-tumor changes.
Identification of pre-tumor changes is extremely important, as it allows one to identify groups at “high risk” for the development of tumors of various locations, prevent the occurrence of a tumor and carry out its early diagnosis.
Among pretumor changes, morphologists distinguish the so-called background changes, manifested by dystrophy, atrophy, and sclerosis, hyperplasia, metaplasia and dysplasia. Foci of hyperplasia, metaplasia and dysplasia are considered as actually precancerous. Among them, the greatest importance has recently been attached to dysplasia.
Precancerous conditions are divided into obligate and facultative precancer. obligate precancer, those. precancer, which almost always results in the development of cancer, is more often associated with a hereditary predisposition. These are congenital polyposis of the colon, xeroderma pigmentosum, neurofibromatosis (Recklinghausen's disease), retinal neuroblastoma, etc. optional precancer include hyperplastic-dysplastic processes, as well as some dysembryoplasia. In addition, there is the so-called latent period of cancer, those. period of existence of the pre-
cancer before cancer develops. For tumors of different localizations, it is different and is sometimes calculated for many years (up to 30-40 years). The concept of “latent period of cancer” applies only to obligate precancer.
Tumor formation or the transition of pretumor changes into a tumor has not been studied enough. Based on experimental data, we can assume the following pattern of tumor development: a) disruption of the regenerative process; b) pretumor changes characterized by hyperplasia and dysplasia; c) malignancy of proliferating cells that occurs in stages; d) the appearance of a tumor germ; e) tumor progression. This scheme is close to L.M. Shabad.
Recently, the theory of the “tumor field”, created by V. Willis (1953) and revealing the staged nature of tumor development, has become widespread. According to this theory, multiple growth points appear in the organ - focal proliferations, which constitute the “tumor field”. Moreover, tumor transformation (malignization) of focal proliferates occurs sequentially from the center to the periphery until the foci of malignancy merge into one tumor node; however, primary multiple growth is also possible. As can be seen, Willis’s theory provides for its appositional growth during the formation of a tumor, i.e. transformation of non-tumor cells into tumor cells and proliferation of the latter. After the “tumor field is spent,” the tumor grows “from itself.” This theory is debatable.
In the formation of a tumor, the role of disruption of the relationship between the epithelium and connective tissue is undoubted. V.G. Garshin (1939) showed that the growth of the epithelium is determined by the structural and functional state of the underlying connective tissue. Normally, the epithelium never grows into mature connective tissue, but only spreads along it. Ingrowth of the epithelium into the underlying tissue is observed in the case of disconnection in the epithelium - connective tissue system.
Histogenesis of tumors
Tumor histogenesis- this is the establishment of its tissue origin.
Determining the histogenesis of a tumor is of great practical importance not only for the correct morphological diagnosis of the tumor, but also for the selection and prescription of reasonable treatment. It is known that tumors of different tissue origin exhibit unequal sensitivity to radiation therapy and chemical drugs.
Tumor histogenesis and histological structure of the tumor are ambiguous concepts. According to the histological structure, the tumor may be close to one or another tissue, although it is not histogenetically related to this tissue. This is explained by the possibility of extreme variability in cell structure during oncogenesis, reflecting morphological catplasia.
The histogenesis of the tumor is established through a morphological study of the structure and comparison of tumor cells with various stages of ontogenetic development of the cells of the organ or tissue in which they develop.
this tumor was gone. In tumors built from differentiated cells, histogenesis is established relatively easily, since the tumor cells remain very similar to the cells of the tissue or organ from which the tumor arises. In tumors made from undifferentiated cells that have lost their resemblance to the cells of the original tissue and organ, it is very difficult and sometimes impossible to establish histogenesis. Therefore, there are still tumors of unknown histogenesis, although the number of such tumors is decreasing thanks to the use of new research methods. Based on electron microscopic data and tissue culture studies, it was shown that the cells of the body during tumor transformation do not lose the specific properties that have developed in phylo- and ontogenesis.
Typically, a tumor occurs in those areas of tissues and organs where cell proliferation occurs most intensively during regeneration - in the so-called proliferative growth centers. Here, less differentiated cells are found (cambial elements - stem, semi-stem cells, blasts, progenitor cells) and more often conditions appear for the development of cellular dysplasia with subsequent transformation into a tumor. Such centers are observed in perivascular tissue, in the basal zone of stratified squamous epithelium, in the crypts of the mucous membranes. The source of the tumor may be areas of epithelial metaplasia. Sometimes a tumor arises from tissue primordia or tissue dystopias that have broken off during embryogenesis.
Depending on their origin from derivatives of various germ layers, tumors are divided into endo-, ecto- And mesodermal. Tumors consisting of derivatives of two or three germ layers are called mixed and belong to the group of teratomas and teratoblastomas (from the Greek. teratos- monster). When tumors occur, it persists law of specific tissue productivity, those. an epithelial tumor develops only from the epithelium, a muscle tumor - from smooth or striated muscles, a nervous tumor - from various cells of the nervous system, a bone tumor - from bone tissue, etc.
Tumor progression
In 1969, L. Foulds, based on experimental oncology data, created a theory tumor progression. According to this theory, a tumor is considered as a formation that continuously progresses through qualitatively different stages, by which are meant inherited changes of an irreversible nature of one or more clearly manifested signs. The acquisition of tumor properties occurs in stages, as a result of the replacement of one population of cells by another, through the selection of cell clones or mutation of tumor cells. This creates the basis for increasing cell autonomy and maximum adaptability to the environment.
According to the theory of tumor progression, the timing of stages and individual properties characterizing a malignant tumor can vary significantly, appear independently of each other and create different combinations of features (independent progression of various tumor symptoms). Tumors of the same type do not achieve the final result in the same way: some tumors acquire their final properties immediately (direct path), others - after going through a series of intermediate stages (indirect path) - During progression, an alternative path of development is selected. At the same time, the development of the tumor along the progression path can never be considered complete.
According to the theory of tumor progression, benign tumors represent one of the phases of progression, which is not always realized in the form of a malignant tumor. Therefore, benign tumors are divided into tumors with high And minimal risk malignancy. The independence of the progression of various tumor features helps explain unpredictability tumor behavior, for example the presence of metastases in a histologically benign tumor with invasive growth. It follows from this that in some cases, with certain tumors, relative independence of such tumor characteristics as cellular atypia, invasive growth and the ability to metastasize may appear. But this is not the rule for most malignant tumors. Foulds's position about the independent progression of various tumor symptoms is not always justified. For example, as a rule, there is a relationship between the level of differentiation of a malignant tumor and its clinical behavior. This is the basis for predicting the course of a tumor based on certain morphological features.
The body's immune response to a tumor
Both forms of immune response occur to tumor cell antigens (tumor antigens): humoral with the appearance of antibodies and cellular with the accumulation of killer T-lymphocytes sensitized against tumor cells. Antitumor antibodies not only protect the body from a tumor, but can also promote its progression, having an enhancing effect (enhancement- phenomenon). Lymphocytes and macrophages, when in contact with tumor cells, can have a cytolytic or cytotoxic effect on them. In addition, macrophages and neutrophils are capable of causing a cytostatic effect, as a result of which DNA synthesis and mitotic activity in tumor cells are reduced. Thus, antitumor immune defense is similar transplant immunity.
Morphologically, the manifestations of the immune reaction to tumor antigens are expressed in the accumulation in the tumor stroma and especially along the periphery of its immunocompetent cells: T- and B-lymphocytes, plasma cells, macrophages. Clinical and morphological observations show
It appears that in cases where the tumor stroma is rich in immunocompetent cells, relatively slow tumor development is observed. Tumors lacking immunocompetent cells in the stroma grow quickly and metastasize early.
In the early stages of tumor development, even before the occurrence of metastases in the lymph nodes regional to the tumor, signs are noted antigenic stimulation. They manifest themselves in hyperplasia of lymphatic follicles with an increase in the size of their reproductive centers, hyperplasia of reticular and histiocytic elements along the sinuses (the so-called sinus histiocytosis), which are considered as an expression of antitumor protection and as a favorable prognostic sign in the absence of tumor metastases.
There is evidence of the participation of the thymus gland in antitumor protection: it carries out immunological surveillance, ensuring the elimination of tumor cells. The dependence of the frequency of tumor development in humans on the condition of this gland has been statistically proven - an increase in tumors when the thymus gland is removed, as well as as its age-related involution increases.
Immune response to tumors insolvent. Among the reasons for this failure, the following are distinguished (Petrov R.V., 1982): 1) the effect of circulating antitumor antibodies that enhances tumor growth (according to the type of enhancement effect); 2) blockade of specific “antitumor” receptors on the surface of lymphocytes by tumor antigens circulating in the blood. The influence of immunological tolerance, the immunosuppressive effect of the tumor itself, an imbalance between the speed of the immune response and tumor growth, genetically determined “unresponsiveness” to certain tumor antigens, and insufficient immune surveillance by the thymus cannot be excluded.
Etiology of tumors (causal genesis)
All the diversity of views on etiology can be reduced to four main theories: 1) viral-genetic, 2) physico-chemical, 3) dysontogenetic, 4) polyetiological.
1. Viral genetic theory assigns a decisive role in the development of neoplasms to oncogenic viruses. The essence of the viral genetic theory (Zilber L.A., 1968) lies in the idea of the integration of the genomes of the virus and a normal cell, i.e. in combining the nucleic acid of the virus with the genetic apparatus of the cell, which will turn into a tumor. Oncogenic viruses can be DNA- and RNA-containing (oncornaviruses). Among exogenous viruses (DNA and RNA containing) the herpes-like Epstein-Barr virus (development of Burkitt's lymphoma), the herpes virus (cervical cancer), the hepatitis B virus (liver cancer) and some others are important in the etiology of human tumors. Along with exogenous ones, endogenous oncogenic ones have also been discovered.
2. Physico-chemical theory reduces the cause of the tumor to the effects of various physical and chemical substances. It was noticed many years ago that cancer occurs under the influence of various irritants. Such observations gave rise to R. Virchow back in 1885 to create the “irritation theory” to explain the causes of cancer. Essentially, the physicochemical theory is a further development of Virchow's theory with a number of additions and changes. Currently, a large group of tumors belonging to the so-called professional cancer. This is lung cancer as a result of filling them with dust containing carcinogenic substances (in cobalt mines), skin cancer of the hands in radiologists, in people working in paraffin production, bladder cancer in those working with aniline dyes. The undoubted influence of smoking on the incidence of lung cancer has been established. There is indisputable evidence of the importance of radioactive isotopes for the occurrence of tumors.
Consequently, tumor development may be associated in many cases with exposure to carcinogenic substances(carcinogens). Attracts special attention chemical carcinogens, among which the most active are polycyclic aromatic hydrocarbons, aromatic amines and amides, nitro compounds, oflatoxins and other waste products of plants and fungi. Chemical carcinogens may be of endogenous origin (Shabad L.M., 1969). Among endogenous chemical carcinogens, the role of tryptophan and tyrosine metabolites is great. It has been proven that chemical carcinogens act on the genetic apparatus of the cell. They cause a number of qualitative changes in the genome of target cells (point mutations, translocations, etc.), which lead to the transformation of cellular proto-oncogenes into active
oncogenes. The latter, through their products - oncoproteins, transform the cell into a tumor cell.
Related to chemical carcinogenesis dyshormonal carcinogenesis. It has been shown that hormonal imbalances play a role in the occurrence and stimulation of tumor growth. An imbalance of tropic hormones is considered as a trigger for carcinogenesis. Participation in this process of estrogens is especially great, which have a direct effect on the target organ and carry out hormonal regulation of proliferative processes in the body.
3. Dysontogenetic theory (disontogenesis- vicious development) was created by Yu. Konheim (1839-1884). According to this theory, tumors arise from embryonic cell-tissue displacements and malformed tissues under the influence of a number of provoking factors. This theory can explain the occurrence of a small number of tumors.
The question of the mechanism of transition of a normal cell into a tumor cell cannot be considered resolved, and yet in the knowledge of this particular question lies the solution to the entire problem of tumor development. Probably, the tumor cell arises as a result of mutation, i.e. sudden transformation of the genome, but changes in the genome of a cell during the process of malignancy can also occur in stages, being extended over time (tumor transformation).
Classification and morphology of tumors
Classification of tumors has been constructed post-histogenetic principle taking into account their morphological structure, localization, structural features in individual organs (organ specificity), benignity or malignancy. This classification was proposed as an international classification by the Committee on Tumor Nomenclature of the International Anticancer Association. According to this classification, 7 groups of tumors are distinguished, and their total number exceeds 200 items.
I. Epithelial tumors without specific localization (organ-nonspecific).
II. Tumors of exo- and endocrine glands, as well as epithelial integuments (organ-specific).
III. Mesenchymal tumors.
IV. Tumors of melanin-forming tissue.
V. Tumors of the nervous system and meninges.
VI. Tumors of the blood system.
VII. Teratomas.
It should be noted that the division of epithelial tumors, according to the classification, into organ-specific and organ-nonspecific is currently not justified, since organ-specific markers have been found for most epithelial tumors. This is of great importance for the morphological diagnosis of tumors.
Below is a description of the most prominent representatives of tumors of each group.
Tumors of this type develop from squamous or glandular epithelium, which does not perform any specific function. This is the epidermis, epithelium of the oral cavity, esophagus, endometrium, urinary tract, etc.
Tumors of this group are divided into benign and malignant; their types are given in Table. 6.
Table 6. Epithelial tumors without specific localization
Source of tumor | Benign tumors | Malignant tumors |
Flat and transitional epithelium | Papilloma | "Cancer in Place", Adenocarcinoma; squamous cell carcinoma with keratinization, without keratinization |
Prismatic and glandular epithelium | Adenoma: acinar, tubular, trabecular, papillary, fibroadenoma, adenomatous polyp | "Cancer in Place", Adenocarcinoma; mucous (colloid) cancer |
Stem cells and progenitor cells epithelium | Cancer: solid, small cell, fibrous, medullary |
Benign tumors
Benign epithelial tumors of this group include papilloma and adenoma.
Papilloma(from lat. papilla- papilla) - a tumor of flat or transitional epithelium (Fig. 100). It has a spherical shape, dense or soft, with a papillary appearance on the surface (like cauliflower or raspberries), ranging in size from a millet grain to a large pea; located above the surface of the skin or mucous membrane on a wide or narrow base. The tumor is built from cells of the growing integumentary epithelium, the number of its layers is increased. In skin papilloma, keratinization of varying intensity can be observed. The stroma is well expressed and grows together with the epithelium. In papilloma, the polarity of the arrangement of cells, complexity, and its own membrane are preserved. Fabric
 Rice. 100. Papilloma
Rice. 100. Papilloma
atypia is represented by uneven development of the epithelium and stroma and excessive formation of small blood vessels.
Papilloma occurs on the skin, as well as on mucous membranes lined with transitional or non-keratinizing squamous epithelium (oral mucosa, true vocal cords, renal pelvis, ureters, bladder).
When injured, the papilloma is easily destroyed and inflamed, and may cause bleeding in the bladder. After removal, papillomas rarely recur, and sometimes (with constant irritation) they become malignant.
Adenoma(from Greek aden- iron, ota- tumor) - a tumor of glandular organs and mucous membranes lined with prismatic epithelium. It looks like a well-demarcated node of soft consistency, the tissue is white-pink when cut, sometimes cysts are found in the tumor. The sizes vary - from a few millimeters to tens of centimeters.
Adenomas of the mucous membranes protrude above their surface in the form of a polyp. They are called adenomatous (glandular) polyps.
Adenoma has an organoid structure and consists of prismatic or cuboidal epithelial cells that form glandular formations, sometimes with papillary outgrowths. The relationship between glandular structures and tumor stroma can be different: if the latter predominates over the glandular parenchyma, they speak of fibroadenoma. The epithelium remains complex and polar, located on its own membrane. Adenoma cells are similar to the cells of the original tissue in morphological and functional respects. Depending on the structural features, in addition to fibroadenoma and adenomatous polyp, they are distinguished: acinar, developing from the alveolar parenchyma of the glands (alveolar adenoma); tubular(Fig. 101), growing from the ducts of glandular structures; trabecular, having a beam structure, and papillary(Fig. 102), represented by papillary growths in cystic formations (cystadenoma). An adenoma can develop into cancer.
 Malignant tumors
Malignant tumors
Malignant tumors developing from poorly differentiated or undifferentiated epithelial cells are designated as Cancer. The tumor usually has the appearance of a node of soft or dense consistency, its boundaries are unclear, sometimes merging with the surrounding tissue. A cloudy liquid is scraped off the whitish surface of the tumor cut - crayfish juice. Cancer of the mucous membranes and skin ulcerates early. The following microscopic forms of cancer: "cancer is in place" (carcinoma in situ); 1shoskokletochny (e1tidermal) with keratinization and without keratinization; adenocarcinoma (glandular); mucous (colloid); solid (trabecular); small cell; fibrous (scirrh); medullary (adenogenic).
"Cancer is in place" or carcinoma in situ(intraepithelial, non-invasive carcinoma) - a form of cancer without invasive (infiltrating) growth, but with pronounced atypia and proliferation of epithelial cells with atypical mitoses (Fig. 103). This form of cancer should be differentiated from severe dysplasia. Tumor growth occurs within the epithelial layer, without moving into the underlying tissue. But non-invasive cancer is only a stage of tumor growth; over time, it becomes infiltrating (invasive).
Squamous cell (epidermal) carcinoma develops in the skin and mucous membranes covered with flat or transitional epithelium (oral cavity, esophagus, cervix, vagina, etc.). In mucous membranes covered with prismatic epithelium, squamous cell carcinoma develops only after previous epithelial metaplasia. The tumor consists of strands of atypical epithelial cells that grow into the underlying tissue, destroy it and form nested clusters in it. Tumor cells can retain the ability to keratinize, then pearl-like formations appear (cancer pearls). With a lower degree of cell differentiation, cancer keratinization does not occur. In this regard, squamous cell carcinoma may be keratinizing and non-keratinizing(Fig. 104, 105).
Adenocarcinoma (glandular cancer) develops from the prismatic epithelium of the mucous membranes and the epithelium of the glands. Therefore, it is found both in the mucous membranes and in the glandular organs. This adenogenic tumor has a structure similar to an adenoma, but unlike an adenoma, in adenocarcinoma there is atypicality of the epithelial cells: they are of different shapes, the nuclei are hyperchromic. Tumor cells form glandular formations of various shapes and sizes, which grow into the surrounding tissue, destroy it, and their basement membrane is lost. Distinguish options adenocarcinomas: acinar- with a predominance of acci in the tumor
 Rice. 103. Cancer is in place (carcinoma in situ)
Rice. 103. Cancer is in place (carcinoma in situ)
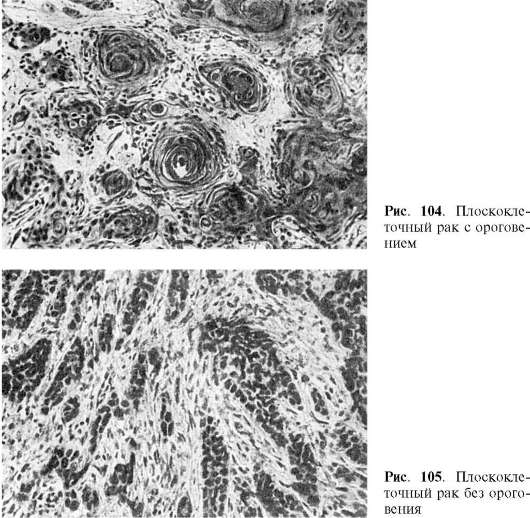 Narny structures; tubular- with a predominance of tubular formations; papillary, represented by atypical papillary growths. Adenocarcinoma can have varying degrees of differentiation.
Narny structures; tubular- with a predominance of tubular formations; papillary, represented by atypical papillary growths. Adenocarcinoma can have varying degrees of differentiation.
Mucous (colloid) cancer- adenogenic carcinoma, the cells of which have signs of both morphological and functional atypia (perverted mucus formation). Cancer cells produce huge amounts of mucus and die in it.
The tumor has the appearance of a mucous or colloidal mass in which atypical cells are found (Fig. 106). Mucous (colloid) cancer is one of the forms of undifferentiated cancer.
Solid cancer(from lat. solidus- single, dense) - a form of undifferentiated cancer with pronounced atypia. Cancer cells are arranged in trabeculae (trabecular cancer), separated by layers of connective tissue. Mitoses are quite frequent in tumor cells. Solid cancer grows quickly and metastasizes early.
 Rice. 106. Mucous (colloid) cancer
Rice. 106. Mucous (colloid) cancer
Small cell cancer- a form of undifferentiated cancer, which consists of monomorphic lymphocyte-like cells that do not form any structures; the stroma is extremely scanty (Fig. 107). There are many mitoses in the tumor, and necrotic changes are often observed. Growth is rapid, metastases occur early. In some cases, it is not possible to establish the histogenesis of the tumor, then they speak of unclassified cancer.
fibrous cancer, or skirr(from Greek scirros- dense), is a form of undifferentiated cancer, represented by extremely atypical hyperchromic cells located among layers and strands of coarse fibrous connective tissue. The main feature of this form of cancer is the clear predominance of stroma over parenchyma. The tumor is highly malignant and early metastases often occur.
Medullary (adenogenic) cancer- a form of undifferentiated cancer; its main feature is the predominance of parenchyma over stroma, which
 the swarm is very small. The tumor is soft, white-pink in color, resembles brain tissue (cerebral cancer). It is represented by layers of atypical epithelial cells and contains many mitoses; grows quickly and undergoes necrosis early; gives early and multiple metastases. In addition to those described, there are mixed
forms of cancer consisting of the rudiments of two types of epithelium (flat and cylindrical), they are called dimorphic crayfish.
the swarm is very small. The tumor is soft, white-pink in color, resembles brain tissue (cerebral cancer). It is represented by layers of atypical epithelial cells and contains many mitoses; grows quickly and undergoes necrosis early; gives early and multiple metastases. In addition to those described, there are mixed
forms of cancer consisting of the rudiments of two types of epithelium (flat and cylindrical), they are called dimorphic crayfish.
Tumors of exo- and endocrine glands as well as epithelial integuments
These tumors are characterized by the fact that they develop from the cells of a particular organ and retain the morphological, but sometimes also functional features inherent in this organ. They are found both in exocrine glands and epithelial integuments, and in endocrine glands.
The types of these tumors are given in table. 7.
Table 7. Tumors of exocrine glands and epithelial integuments
Source of tumor | Benign tumors | Malignant tumors |
Liver Hepatocytes | Adenoma (hepatoma) | Hepatocellular carcinoma |
Kidneys Tubular epithelium Metanephrogenic tissue | Adenoma | Renal cell carcinoma Nephroblastoma |
Breast Epithelium of alveoli and excretory ducts Epidermis of the nipple and areola; ductal epithelium | Fibroadenoma (pericanalicular, intracanalicular) | Lobular “carcinoma in situ”, ductal “carcinoma in situ” Paget's disease (cancer) |
Uterus Chorion membrane | Hydatidiform mole | Destructive (malignant) hydatidiform mole; chorionepithelioma (chorionic carcinoma) |
Leather Epithelium of sweat gland ducts Epithelium of the secretory sections of the sweat glands Epithelium of hair follicles Epithelium of different parts of skin appendages | Syringoadenoma Hidradenoma Trichoepithelioma | Cancer Cancer Basal cell carcinoma |
Liver
Hepatocellular adenoma (hepatoadenoma)- a benign tumor, built from hepatocytes that form trabeculae. It occurs in the form of one or more nodes.
Hepatocellular (hepatocellular) cancer may be represented by one large nodule covering almost the entire lobe of the liver (massive form), several isolated nodes (nodular form) or nodules scattered in the liver tissue (diffuse form). The tumor is built from atypical hepatocytes that form tubules, acini or trabeculae (tubular, acinar, trabecular, solid cancer). The stroma is scanty with thin-walled blood vessels.
Kidneys
TO benign tumors include adenomas, malignant - variants of renal cell carcinoma.
Among kidney adenomas, dark cell (basophilic), clear cell (hypernephroid) and acidophilic are distinguished.
Dark cell (basophilic) adenoma may have the structure of a tubular, solid adenoma or cystopathilloma. Sometimes it reaches the size of the kidney itself. Clear cell (hypernephroid) adenoma usually small in size, surrounded by a capsule, yellow in section, sometimes with hemorrhages; built from large polymorphic light, lipid-rich cells. Acidophilic adenoma- a rare tumor, reaches large sizes, has a tubular, solid or papillary structure. Tumor cells are polygonal, light, with acidophilic granularity.
Renal cell (hypernephroid) cancer has several variants: clear cell (hypernephroid), granular cell; glandular (renal adenocarcinoma); sarcoma-like (spindle and polymorphic cell); mixed cell carcinoma. Each type of kidney cancer (except sarcoma-like) can have a different degree of differentiation. The most typical are clear cell and glandular variants.
Clear cell (hypernephroid) cancer- the most common malignant tumor of the kidney. It is represented by a node of soft and variegated tissue, consisting of lipid-containing light polygonal and polymorphic cells with numerous mitoses. Cancer cells form alveoli and lobules, glandular and papillary structures, separated by scant stroma with sinusoidal vessels; necrosis and hemorrhage are typical. Characteristically, the tumor invades the pelvis and grows through the veins (“tumor blood clots”). Hematogenous metastases occur early in the lungs, bones, liver, and opposite kidney.
Glandular cancer (renal adenocarcinoma) has the appearance of a soft variegated knot. The tumor consists of tubular and papillary structures; its cells are atypical, with hyperchromic nuclei. Cancer invades the kidney tissue and gives hematogenous metastases.
Nephroblastoma (embryonic nephroma, embryonal kidney cancer, Wilms tumor)- malignant tumor; most common in children (see Diseases of childhood).
Breast
Breast tumors are very diverse and often develop against the background of dishormonal benign dysplasia.
Benign tumors include fibroadenoma, which has the appearance of an encapsulated unit of dense consistency. Proliferation of alveoli and intralobular ducts is characteristic. Connective tissue can overgrow intralobular ducts (pericanalicular fibroadenoma- rice. 108) or grow into them (intracanalicular fibroadenoma- see fig. 108). Rarely found leaf-shaped (phylloid) tumor.
Types of breast cancer include non-infiltrating lobular and intraductal cancer, Paget's disease.
Non-infiltrating lobular carcinoma (lobular “carcinoma in situ”) arises multicentrically, has solid And glandular options (Fig. 109). Develops in an unchanged lobule or against the background of dishormonal benign dysplasia. It may progress to an invasive form of cancer.
Non-infiltrating intraductal carcinoma (ductal carcinoma in situ) can be papillary, acne-like and cribriform. Papillary cancer grows, filling the lumen of the dilated ducts, and does not go beyond their limits. Acne cancer occurs multicentrically, but is usually limited to one segment of the gland. Intraductal growths of anaplastic epithelium (Fig. 110) undergo necrosis. These necrotic, sometimes calcified, tumor masses are squeezed out
 Rice. 108. Fibroadenoma of the breast:
Rice. 108. Fibroadenoma of the breast:
a - pericanalicular; b - intracanalicular
when cut, it appears from the ducts in the form of whitish crumbling plugs (which is why the cancer is called acne-like). Intraductal cancer becomes invasive. Cribose cancer histologically it has the appearance of a lattice due to the formation of gaps at the site of dead cells.
Paget's disease The mammary gland is characterized by three signs: eczematous lesions of the nipple and areola; the presence of large, light cells in the epidermis of the nipple and areola; cancerous lesion of the mammary duct. In the thickened and somewhat loosened epidermis, peculiar light tumor cells are found, called cells Paget. They lack intercellular bridges and are located in the middle sections of the germinal layer of the epidermis, but can also reach the stratum corneum. Paget cells never invade the dermis. Cancer develops from the epithelium of both large and small ducts and has the structure of scirrus, acne or cribriform carcinoma.
There is an opinion (Golovin D.I., 1981) that Paget’s disease develops not from one small focus of cells, but multicentrically, in a large tumor field, consisting of three sections: the epidermis of the nipple and areola, the mouths of large ducts and the deeper small ducts of the mammary gland . Tumor progression is manifested by appositional growth and sequential involvement of new epithelial structures in the process. According to this view, Paget's cells are altered and malignant epithelial elements of the germ layer.
 Rice. 109. Lobular breast cancer
Rice. 109. Lobular breast cancer
 Rice. 110. Ductal breast cancer
Rice. 110. Ductal breast cancer
Uterus
Epithelial tumors of the uterus are destructive (malignant) hydatidiform mole and chorionepithelioma (chorionic carcinoma).
Destructive (malignant) hydatidiform mole characterized by ingrowth of chorionic villi into the veins of the uterus and pelvis. Secondary foci of tumor growth appear in the uterus and other organs (vagina, lungs). Chorionic villi are small in size; syncytial cells predominate in the proliferating trophoblast. Destructive hydatidiform mole transforms into chorionepithelioma in half of the cases.
Chorionepithelioma (chorionic carcinoma)- a malignant trophoblast tumor that develops from the remnants of the placenta after abortion, tubal pregnancy, childbirth, and especially often during destructive hydatidiform mole. The tumor has the appearance of a variegated spongy node in the myometrium. Previously, this tumor was called deciduoma, as it was assumed that it develops from the decidual tissue of the pregnant uterus. In 1886, Moscow pathologist M.N. Nikiforov and almost simultaneously the Swiss pathologist Marchand established that the tumor develops from the epithelium of the chorionic villi, i.e. the fetus, not the mother. The tumor was named chorionepithelioma. It consists of cyto- and syncytiotrophoblast elements (Fig. 111): light epithelial Langhans cells, among which there are many giant dividing and polymorphic dark syncytium cells. There is no stroma in the tumor, the vessels look like cavities lined with tumor cells, and therefore hemorrhages are frequent. Tumor cells easily penetrate the blood and give hematogenous metastases, primarily to the lungs. Chorionepithelioma is hormonally active: its development is accompanied by the release of the hormone gonadotropin, which is found in the urine. In very rare cases, chorionepithelioma can be of teratogenic origin, which explains its development in women in the ovary and in men in the testicle, mediastinum, and bladder wall. Such chorionepitheliomas are called ectopic.
 Leather
Leather
Skin tumors are very numerous and arise both from the epidermis and from skin appendages: sweat and sebaceous glands, glands of hair follicles. These tumors are divided into benign, tumors with locally destructive growth and malignant. The most important of them are syringoadenoma, hidradenoma, trichoepithelioma and basal cell carcinoma (basalioma).
Syringoadenoma- a benign tumor of the epithelium of the sweat gland ducts. Distinguish papillary And tubular form. The first is characterized by the formation of papillae covered with double-layered epithelium, the second - randomly located tubules also lined with double-layered epithelium. Hidradenoma- a benign tumor of the secretory epithelium of the sweat glands with papillary outgrowths of the epithelium. Trichoepithelioma- a benign tumor of hair follicles or their embryonic elements. Characterized by malformed hair follicles and squamous epithelial cysts filled with horny substance.
Basal cell carcinoma (basal cell carcinoma)- a tumor with local destructive growth, recurs, but does not metastasize; localized most often on the neck or face; looks like a plaque or deep ulcer (ulcus rodens). The tumor is often multiple. Constructed of small round, oval or spindle-shaped cells with a narrow rim of basophilic cytoplasm (dark cells), reminiscent of the basal cells of the epidermis, but lacking intercellular bridges. The cells are arranged in cords or nests, in which formations similar to skin appendages may appear. Basalioma is one of the most common skin tumors.
Among malignant tumors developing from skin appendages, there are sweat gland cancer, sebaceous gland cancer And hair follicle cancer. These tumors are rare.
End of table. 8
Source of tumor | Benign tumors | Malignant tumors |
Testicles |
||
Sex cells | Seminoma |
|
Glandulocytes (Leydig cells) | Leydig cell tumor | |
Sustentocytes (Sertoli cells) | Sertoli cell tumor | |
Thyroid gland |
||
Cells A and B | Follicular adenoma | Follicular cancer; papillary cancer; undifferentiated cancer |
Cells C | Adenoma solid | Solid cancer with stromal amyloidosis (medullary Cancer) |
Parathyroid glands |
||
Chief cells | Adenoma | Cancer |
Adrenal glands |
||
Cortical cells | Adrenocortical adenomas | Adrenocortical cancer |
Cells of the medulla | Pheochromocytoma | Malignant pheochromocytoma (pheochromoblastoma) |
Thymus gland |
||
Epithelial cells | Timoma (cortical cell, medullary cell mixed cell, granulomatous) |
|
Cancer |
||
Pituitary | Adenoma: chromophobic, eosinophilic, basophilic | Cancer |
Pineal gland | Pinealoma | |
Pancreas |
||
β-Cells | β-Insuloma | |
α-Cells | α-Insuloma | Malignant insulinoma |
G-Cells | G-Insuloma | |
Gastrointestinal tract |
||
Enterochromaffin cells | Carcinoid | Malignant carcinoid |
Ovaries
Ovarian tumors are diverse and, depending on their origin, are divided into epithelial, sex cord stromal tumors and germ cell tumors; they can be benign or malignant. Below is a description of some of these tumors.
Serous cystadenoma- epithelial benign tumor of the ovary, often unilateral. It is a cyst, sometimes large in size, with a smooth surface. It has a whitish appearance when cut and consists of one or more cysts filled with serous fluid. The cysts are lined with heterogeneous epithelium (sometimes it resembles tubal or cervical epithelium), with papillary growths; in these cases they talk about papillary cystadenoma.
Mucinous cystadenoma (pseudomucinous cystoma)- benign epithelial tumor, unilocular or multilocular, usually unilateral. It can reach very large sizes and weights (up to 30 kg). The cysts are lined with tall prismatic epithelium, resembling intestinal epithelium and secerating mucus (mucoid); the formation of papillary epithelial outgrowths into the lumen of the cyst is possible (papillary mucinous cystadenoma). In some cases, the wall of the mucinous cyst ruptures, its contents pour into the abdominal cavity, and develops pseudomyxoma peritonei. In this case, implantation of cyst cells along the peritoneum is possible; A large amount of mucus secreted by cells accumulates in the abdominal cavity.
Serous cystadenocarcinoma- epithelial malignant tumor, one of the most common forms of ovarian cancer. Papillary growths of anaplastic epithelium predominate; foci of a solid or adenomatous structure often appear. Tumor cells grow into the wall of the cyst, spread over its surface and move to the peritoneum.
Pseudomucinous cystcarcinoma (cancer of a pseudomucinous cyst)- malignant mucinous tumor of the ovaries (Fig. 112). Consists of multilayered layers of atypical cells, the mucus-forming function of which is reduced; cells form glandular, solid, cribriform structures; Necrosis of tumor tissue is characteristic.
 Rice. 112. Pseudomucinous ovarian cyst with progression to cancer
Rice. 112. Pseudomucinous ovarian cyst with progression to cancer
Tecoma- benign tumor of the ovarian sex cord stroma; often one-sided, reaches large sizes, dense, yellow in color. More often observed over the age of 50 years. The tumor may be hormonally inactive, in which case its structure resembles a fibroma and consists of intertwined bundles of spindle-shaped cells. With hormonally active thecoma, tumor cells accumulate lipids, become round, light-colored, and resemble epithelium. They are located diffusely or in nests. A well-developed network of capillaries appears between the tumor cells. Hormonally active thecoma, producing estrogens, manifests itself in girls as premature ripening, in young women as menstrual disorder, in older women as metrorrhagia (irregular uterine bleeding). Hyperplasia and decidual transformation of the uterine mucosa are possible. Thecoma malignant- a rare tumor, characterized by cellular atypia, built from round, spindle-shaped and polymorphic cells, reminiscent of sarcomatous ones. Hormonal activity is rare.
Granulosa cell tumor (folliculoma)- a benign tumor of the sex cord of the ovary, often unilateral, is a node with a lumpy surface, gray-yellow in section, with foci of hemorrhage. The source of tumor growth is granulosis. The main element of the tumor is small round cells with a basophilic nucleus and a thin rim of cytoplasm. The cells form trabecular or adenomatous structures. This is a hormonally active tumor; high levels of estrogen are found in the blood and urine. Hormonal influence is manifested by hirsutism (increased hair growth), premature puberty, amenorrhea, and glandular-cystic endometrial hyperplasia. Malignant granulosa cell tumor (cancer) retains the ability to produce estrogen, but the cells lose their monomorphism and become polymorphic. Meet combined(dimorphic) granulosatheca cell malignant tumors.
Dysgerminoma- malignant germ cell tumor of the ovary. It is rare in girls and women, and sometimes develops against the background of infantilism. It has the appearance of a rather dense large node, occurring more often in one ovary; on the section it is gray with areas of hemorrhage. Constructed of large cells with a centrally located nucleus; they form alveolar accumulations, delimited by layers of connective tissue containing many lymphocytes. The tumor metastasizes early to the lymph nodes. It is believed that the tumor is formed from the germ cells of the male gonad primordium; its histological structure resembles testicular seminoma.
Testicles
Testicular tumors are relatively rare, but are very diverse depending on the nature of the tissue germ from which they develop. In the testicle there are distinguished: germ cell tumors, which arise
from immature germ cells; tumors from gonadal stroma cells; tumors arising simultaneously from germ cells and gonadal stroma cells; tumors from the membranes of the testicle and from the tissue of the appendages.
Seminoma (dysgerminoma)- germ cell malignant and the most common testicular tumor. It is observed at the age of 40-50 years, often with cryptorchidism. It consists of one or more nodes of elastic white tissue with foci of necrosis. It is represented by a cluster (strands and layers) of round, large, glycogen-containing light cells; in the nuclei, chromatin is distributed unevenly, there are many atypical mitoses. The stroma consists of delicate fibrous connective tissue with extensive infiltrates of lymphocytes, plasma cells, and sometimes eosinophils (Fig. 113). The first metastases appear in the peri-aortic and iliac lymph nodes, hematogenous metastases - in the lungs, liver, kidneys, and pleura.
Gonadal stromal tumor may arise from glandulocytes (Leydig cells) and is called Leydig cell tumors, or Leydigoma, a tumor of sustentocytes (Sertoli cells) is called Sertoli cell tumor. Both types of tumors are rare and have a benign course. A Leydig cell tumor causes premature puberty in children and gynecomastia in adults; Sertoli cell tumor manifests itself as feminization, gynecomastia.
Thyroid gland
Tumors of the thyroid gland are diverse, since each of its cells (A, B and C) can be a source of development benign (adenoma) And malignant (cancer) tumors.
Adenomas The thyroid gland is varied. Follicular adenoma develops from A- and B-cells, is similar in structure to the thyroid gland, consists of small (microfollicular) and larger (macrofollicular) follicles. Solid adenoma originates from C cells that secrete calcitonin. The tumor cells are large, with light oxyphilic cytoplasm, grow among filled collo-
 Rice. 113. Seminoma
Rice. 113. Seminoma
idom of follicles. In cases where cystic formations with branching papillary structures appear in the tumor, they speak of papillary adenoma thyroid gland. The presence of papillary structures in an adenoma is an unfavorable sign regarding malignancy.
Thyroid cancer develops most often from a previous adenoma. Histologically, it is represented by several types.
Follicular cancer arises on the basis of follicular adenoma. It is represented by atypical follicular cells growing into the capsule and walls of blood vessels. Hematogenous bone metastases often occur. One of the variants of this tumor is proliferating struma of Langhans, in which there is no pronounced cellular atypia, but there is a tendency to infiltrating growth and metastasis. Follicular cancer from A cells has a relatively favorable course and prognosis; metastases occur late in the disease. Cancer from B cells It proceeds slowly, but its prognosis is less favorable, since metastases appear early in the lungs and bones.
Papillary cancer It ranks first in frequency among all malignant tumors of the thyroid gland. Consists of cavities of varying sizes, lined with atypical epithelium and filled with papillae emanating from the cyst wall; In some places, the papillae grow into the wall of the cavities and the tumor capsule. One type of papillary cancer that develops from A cells is sclerosing microcarcinoma, or microcarcinoma in the rumen, discovered by chance during microscopic examination.
Solid (medullary) cancer with stromal amyloidosis histogenetically associated with C-cells, which is proven by the presence of calcitonin in the tumor and the similarity of the ultrastructure of tumor cells with C-cells. In the tumor stroma, amyloid is detected, which is formed by tumor cells (APUD-amyloid).
Undifferentiated cancer develops mainly in older people, more often in women. Built from nests and randomly arranged cells of different sizes, sometimes very small (small cell cancer) or giant (giant cell carcinoma).
Parathyroid glands
Benign tumor - adenoma parathyroid glands - develops from chief cells. Atypical cells with hyperchromic nuclei form acini, trabeculae, cysts with papillary growths. The tumor is hormonally active, accompanied by hyperparathyroidism, which underlies fibrous osteodystrophy(cm. Diseases of the musculoskeletal system).
Parathyroid cancer is rare and does not have any specific morphological features.
Adrenal glands
Hormone-active adrenal tumors develop from cells of the cortex or medulla. They can be benign or malignant.
Benign Tumors of the adrenal cortex are adrenocortical adenomas, which can have a different structure. Clear cell adrenocortical adenoma, single or multiple, built from large cells with light cytoplasm containing lipids. Manifested by hyperaldosteronism (Conn's syndrome), therefore this adenoma is also called aldosteroma.
Dark cell adrenocortical adenoma consists of small dark cells containing lipofuscin and forming anastomosing cords. Manifests androgenic activity (androsteroma), signs of virilism (masculinity, from lat. vir- man), less often - Cushing's syndrome. Mixed adrenocortical adenoma, consisting of light and dark cells, manifests itself as hypercortisolism (Cushing's syndrome), which is why it is called corticosteroma. Glomerulosa cell adenoma built from foam cells that do not contain lipids; its structure resembles the zona glomerulosa of the adrenal gland. Clinical manifestations are associated with excess production of mineralocorticoids.
Malignant tumor of the cortex adrenal glands - adrenocortical cancer. It has a polymorphic structure. Characterized by invasive growth, predominantly hematogenous metastasis. Rarely seen.
Benign brain tumor adrenal glands is called pheochromocytoma (from the Greek. phaios- dark and chroma- coloring). Pheochromocytoma- a hormonally active tumor, usually one-sided, gray-red or brown in color when cut. Constructed of polymorphic cells with light cytoplasm (chromaffin tissue cells), which secrete large amounts of catecholamines, which causes increased blood pressure and a number of other disorders.
Malignant brain tumor adrenal glands - malignant pheochromocytoma (malignant pheochromoblastoma)- characterized by pronounced cellular atypia, is extremely rare.
Thymus gland (thymus)
Tumors of the thymus gland - thymomas - develop from cortical and medullary epithelial cells. They are benign and malignant. They look like one or more encapsulated nodes; they can grow into the organs of the anterior mediastinum. The clinical course is asymptomatic or with manifestations of compression of surrounding organs, as well as autoimmune diseases (myasthenia gravis, systemic lupus erythematosus, rheumatoid arthritis, etc.) or immunodeficiency syndromes.
Depending on the degree of infiltration of tumor tissue by T-lymphocytes, thymomas with minimal, moderate And a significant number of lymphocytes.
Morphologically There are 4 types of thymomas (Mulleg-Hermelink H., 1986). Cortical cell thymoma develops from the cortical epithelium, as well as from the cells of thymic bodies, is built from large polygonal cells with rounded light nuclei. The tumor is often malignant (Fig. 114).
 Rice. 114. Malignant cortical cell thymoma with minimal lymphocytes
Rice. 114. Malignant cortical cell thymoma with minimal lymphocytes
Medullary cell thymoma comes from the epithelium of the medulla, can be formed by elongated cells with oval dark nuclei forming nests and cords (spindle cell thymoma). The tumor is usually benign.
Mixed cell thymoma characterized by a combination of morphological characteristics of the two previous species.
Granulomatous thymoma among the tumor cells there are atypical multinucleated epithelial cells, similar to Berezovsky-Sternberg cells in lymphogranulomatosis. For malignant tumors of the thymus, built from atypical cells similar to squamous or glandular epithelium, they speak respectively about squamous cell carcinoma or adenocarcinoma of the thymus.Pituitary
Morphologically differentiate chromophobic, eosinophilic And basophilic adenoma. They may have hormonal activity and be accompanied by the development of a characteristic syndrome.
Among hormonally active pituitary adenomas there are: somatotropic(eosinophilic adenoma); prolactin(chromophobe or eosinophilic adenoma); adenoma of ACTH-secreting cells adenoma of cells secreting thyroid-stimulating hormone(chromophobe or basophilic adenoma); adenoma of follicle-stimulating hormone-secreting cells(chromophobe adenoma), which is extremely rare (in eunuchs).
Meet malignant analogs (cancer) of pituitary adenomas.
Pineal gland
Organ-specific tumor of the pineal gland - pinealoma- built from glandular epithelium and neuroglia. Causes metabolic and hormonal disorders in the body. Rarely seen.
Pancreas
Tumors of the islet apparatus of the pancreas belong to tumors of the APUD system, or apudomam.
Islet cell adenomas are called insulinomas. They are hormonally active. There are three types of insulomas: 1) insuloma from β-cells that produce insulin (β-insuloma); 2) insulin from α-cells, producing
glucagon (α-insuloma); 3) insulinoma from G-cells synthesizing gastrin (G-insuloma). β-Insuloma is manifested by hyperinsulinism and hypoglycemia, α-insuloma by paroxysmal or persistent hyperglycemia, G-insuloma by the development of ulcers in the stomach and duodenum (ulcerogenic insulinoma), which is the essence of Zollinger-Ellison syndrome.
Malignant variants are called insulin malignant insulinomas. They can maintain their hormonal activity.
Gastrointestinal tract
A kind of tumor occurs in the mucous membrane of the stomach and intestines - carcinoid, which develops from Kulchitsky enterochromaffin cells. These cells are representatives of the APUD system, therefore the carcinoid is classified as apudom. More often, various parts of the intestine (appendix) are affected, less often the stomach. The tumor is usually small in size, yellow in section, and consists of nests and strands of polygonal cells separated by layers of connective tissue (Fig. 115). The cells contain birefringent lipids, as well as serotonin grains, and therefore produce chromaffin and argentaffin reactions. Carcinoid may be accompanied larcinoid syndrome(increased blood pressure, heart damage, etc.). In rare cases, carcinoid can become malignant - malignant carcinoid and give metastases.
 Rice. 115. Carcinoid
Rice. 115. Carcinoid
Mesenchymal tumors
In ontogenesis, mesenchyme gives rise to connective tissue, blood vessels, muscles, tissues of the musculoskeletal system, serous membranes, and the hematopoietic system. Under certain conditions, all its derivatives can serve as a source of tumor growth. Mesenchymal tumors can develop from connective (fibrous), adipose, muscle tissue, hematopoietic and lymphatic vessels, synovial, mesothelial and bone tissue. They can be benign or malignant. The main types of this group of tumors are given in Table. 9.
Table 9. Mesenchymal tumors
Source of tumor | Benign tumors | Malignant tumors |
Connective (fibrous) tissue | Fibroma: dense, soft, desmoid | Fibrosarcoma: differentiated, undifferentiated |
Adipose tissue | Lipoma Hibernoma | Liposarcoma Malignant hibernoma |
Muscle tissue | Leiomyoma Rhabdomyoma Granular cell tumor | Leiomyosarcoma Rhabdomyosarcoma Malignant granular cell tumor |
Blood vessels | Hemangioma: capillary, venous, cavernous; benign hemangiopericytoma Glomus tumor (glomus angioma) | Angiosarcoma: malignant hemangioendothelioma, malignant hemangiopericytoma |
Lymphatic vessels | Lymphangioma | Lymphangiosarcoma (malignant lymphangioendothelioma) |
Synovial membranes | Benign synovioma | Malignant synovioma |
Mesothelial tissue | Malignant mesothelioma |
|
Bone tissue | Osteoma, benign osteoblastoma Chondroma, benign chondroblastoma | Osteosarcoma Chondrosarcoma |
Benign tumors
The types of benign mesenchymal tumors are diverse (see Table 9).
Fibroma- a tumor of connective (fibrous) tissue. It is usually represented by a node of differentiated connective tissue, bundles of fibers and vessels are located in different directions (Fig. 116). There are two types of fibroids: dense with a predominance of collagen bundles over cells and soft, consisting of loose connective tissue with a large number of cells such as fibroblasts and fibrocytes.
The localization of the tumor is very diverse. It is more common in the skin, uterus, mammary gland and other organs. On the skin, fibroma sometimes sits on a stalk. When localized at the base of the skull, in the spinal canal or in the orbit, fibroids can cause serious consequences.
Desmoid- a peculiar type of fibroma, most often localized in the anterior wall of the abdomen. Built like a dense fibroma,
 Rice. 116. Fibroma
Rice. 116. Fibroma
but often shows a tendency towards infiltrating growth. After removal it sometimes recurs. It occurs mainly in women, and tumor growth increases during pregnancy.
Dermatofibroma (histiocytoma)- a tumor in the form of a small node, yellow or brown in color when cut; occurs more often on the skin of the legs. It consists of many capillaries, between which there is connective tissue in the form of rhythmic structures containing cells such as fibroblasts, histiocytes - macrophages and fibrocytes. Characterized by large and multinucleated giant cells containing lipids and hemosiderin (Touton cells).
Lipoma- single or multiple tumors of adipose tissue. It has the appearance of a node(s), built from fat lobules of irregular shape and unequal sizes. It is found wherever there is adipose tissue. Sometimes a lipoma does not have clear boundaries and infiltrates the intermuscular connective tissue, causing muscle atrophy (intramuscular Naya, or infiltrating lipoma).
Hibernoma- a rare brown fat tumor. It has the appearance of a node with a lobular structure; consists of cells and lobules formed by round or polygonal cells with granular or foamy cytoplasm due to the presence of fat vacuoles (multilocular fat cells).
Leiomyoma- smooth muscle tumor. Bundles of smooth muscle cells are located chaotically, the stroma is formed by layers of connective tissue in which blood and lymphatic vessels pass. If the stroma is overdeveloped, the tumor is called fibroids. Leiomyoma can reach large sizes, especially in the uterus (Fig. 117). Often there are secondary changes in the form of necrosis, cyst formation, and hyalinosis.
Rhabdomyoma- a tumor of striated muscle cells resembling embryonic muscle fibers and myoblasts. Often occurs due to tissue development disorders and is combined with other por-
 Rice. 117. Nodule of fibroids in the uterus (in section)
Rice. 117. Nodule of fibroids in the uterus (in section)
kami of development (see Diseases of childhood). This applies, for example, to myocardial rhabdomyomas, which usually occur with disorders of brain development (the so-called tuberous sclerosis).
Granular cell tumor(Abrikosov tumor) is usually small in size, has a capsule, and is localized in the tongue, skin, and esophagus. It consists of compactly located round-shaped cells, the cytoplasm of which is fine-grained and does not contain fat (Fig. 118). A.I. Abrikosov, who first described this tumor (1925), believed that it develops from myoblasts (myoma from myoblasts). However, in recent years, an opinion has been expressed about its histiocytic or neurogenic origin.
Hemangioma- a collective concept that includes neoplasms of a disembryoplastic and blastomatous nature. There are capillary, venous, cavernous hemangiomas and benign hemangiopericytoma. Capillary hemangioma localized in the skin, mucous membranes of the gastrointestinal tract, liver; more often observed in children. It is represented by a red or bluish node with a smooth, tuberous or papillary surface; consists of branching capillary-type vessels with narrow lumens; characterized by multinucleation of endothelial cells. The stroma is loose or fibrous. Venous hemangioma has the appearance of a node, consists of vascular cavities, the walls of which contain bundles of smooth muscles and resemble veins. Cavernous hemangioma found in the liver, skin, spongy bones, muscles, gastrointestinal tract, and brain. It has the appearance of a red-blue spongy node, well demarcated from the surrounding tissue. It consists of large vascular thin-walled cavities (cavities), lined with endothelial cells and filled with liquid or clotted blood (Fig. 119). Benign hemangiopericytoma- vascular tumor with predominant localization in the skin and intermuscular layers of the extremities. Built from chaotically
 located capillaries surrounded by muffs of proliferating pericytes; between the cells there is a rich network of ar-gyrophilic fibers.
located capillaries surrounded by muffs of proliferating pericytes; between the cells there is a rich network of ar-gyrophilic fibers.
Glomus tumor(glomus angioma) is localized in the skin of the hands and feet, mainly on the fingers; consists of slit-like vessels lined with endothelium and surrounded by muffs of epithelioid (glomus) cells; the tumor is rich in nerves.
Lymphangioma develops from lymphatic vessels growing in different directions and forming a node or diffuse thickening of the organ (in the tongue - macroglossia, in the lip - macrocheilia). A section of the tumor shows cavities of varying sizes filled with lymph.
Benign synovioma arises from the synovial elements of tendon sheaths and tendons. It is built from polymorphic large cells located in the form of alveoli and multinucleated giant cells (gigantoma). Between the cells there are bundles of connective tissue, often hyalinized, fibers; there are few vessels. Xanthoma cells are sometimes found in the central part of the tumor.
Benign mesothelioma- tumor of mesothelial tissue. Usually presented as a dense node in the serous membranes (pleura) and is similar in structure to fibroma (fibrous mesothelioma).
Among bone tumors there are bone-forming And cartilaginous tumors, giant cell tumor And bone marrow tumors.
Benign bone-forming tumors are osteoma and benign osteoblastoma, cartilage-forming tumors are
chondroma and benign chondroblastoma. Osteoma can develop in both tubular and cancellous bones; most often in the bones of the skull. Extraosseous osteoma occurs in the tongue and breast. Distinguish spongy And compact osteoma. Spongy osteoma built from randomly located bone beams, between which fibrous connective tissue grows; compact osteoma is an array of bone tissue devoid of the usual osteoid structure.
Benign osteoblastoma consists of anastomosing small osteoid and partially calcified bone beams (osteoidosteoma), between which there are many vessels and cellular-fibrous tissue with multinucleated osteoclasts.
Chondroma- a tumor arising from hyaline cartilage. It is dense, and when cut it looks like hyaline cartilage. Constructed from randomly arranged mature cells of hyaline cartilage, enclosed in the ground substance, it can reach large sizes. The most common localization is the hands and feet, vertebrae, sternum, and pelvic bones. If the tumor is localized in the peripheral parts of the bone, it is called ecchondroma, in the central parts of the bone - enchondroma.
Benign chondroblastoma differs from chondroma in that chondroblasts and chondroid interstitial substance are found in it; the reaction of osteoclasts is more pronounced.
Giant cell tumor- cm. Diseases of the dental system and oral cavity organs.
Malignant tumors
Malignant mesenchymal tumors consist of immature cells derived from mesenchyme (see Table 9). They are distinguished by cellular atypia, sometimes expressed to such an extent that it is impossible to establish the true origin of the tumor.
In such cases, histochemistry, immunomorphology, electron microscopy and tissue culture are helpful.
A malignant mesenchymal tumor is designated by the term “sarcoma” (from the Greek. sarcos- meat). When cut, it resembles fish meat. Sarcoma usually metastasizes by hematogenous route.
Fibrosarcoma- a malignant tumor of fibrous connective tissue, found most often on the shoulder and thigh. In some cases, it is delimited and looks like a node, in others its boundaries are erased, the tumor infiltrates the soft tissues. Consists of immature fibroblast-like cells and collagen fibers. Depending on the degree of maturity and the relationship of the cellular and fibrous elements of the tumor, differentiated and poorly differentiated fibrosarcomas are distinguished. Differentiated fibrosarcoma has a cellular fibrous structure (fibrous cell sarcoma- rice. 120), and the fibrous component predominates over the cellular one. Poorly differentiated fibrosarcoma consists of immature polymorphic cells with an abundance of mitoses (cell sarcoma- see fig. 120), it has a more pronounced
 Rice. 120. Fibrosarcoma:
Rice. 120. Fibrosarcoma:
a - differentiated (fibrous cell sarcoma); b - poorly differentiated (cellular sarcoma)
malignancy and more often gives metastases. Sarcomas of round or polymorphic cells may have an unclear histogenesis, then they speak of an unclassified tumor.
Dermatofibroma protuberans (malignant histiocytoma) differs from dermatofibroma (histiocytoma) in the abundance of fibroblast-like cells with mitoses. It is characterized by slow infiltrating growth, relapses, but rarely produces metastases.
Liposarcoma (lipoblastic lipoma)- malignant tumor of adipose tissue. It is relatively rare, reaches large sizes, and has a greasy surface on the cut. Constructed of lipocytes of varying degrees of maturity and lipoblasts. There are several types of liposarcomas: predominantly highly differentiated; predominantly myxoid (embryonic); predominantly round cell; predominantly polymorphic cellular.
Liposarcoma grows relatively slowly and does not metastasize for a long time.
Malignant hibernoma It is distinguished from hibernoma by extreme polymorphism of cells, among which giant cells are found.
Leiomyosarcoma- malignant tumor of smooth muscle cells (malignant leiomyoma). It differs from leiomyoma by pronounced cellular and tissue atypia, a large number of cells with typical and atypical mitoses. Sometimes atypia reaches such a degree that it is impossible to establish the histogenesis of the tumor.
Rhabdomyosarcoma- malignant tumor of striated muscles (malignant rhabdomyoma). The structure is extremely polymorphic, the cells lose their resemblance to striated muscles. However, identification of individual cells with cross-striations, as well as the results of immunohistochemical studies using specific serum, make it possible to verify the tumor.
Malignant granular cell tumor- a malignant analogue of fibroids from myoblasts, or Abrikosov tumor (malignant myoblastoma), is extremely rare. It is similar to malignant rhabdomyoma and contains atypical cells with granular cytoplasm.
Angiosarcoma- a malignant tumor of vascular origin, rich in atypical cells of either an endothelial or periocytic nature (Fig. 121). In the first case we talk about malignant hemangioendothelioma, in the second - about malignant hemangiopericytoma. The tumor is highly malignant and metastasizes early.
Lymphangiosarcoma occurs against the background of chronic lymphostasis and is represented by lymphatic clefts with proliferating atypical endothelial cells (malignant lymphangioendothelioma).
Synovial sarcoma (malignant synovioma) observed in large joints. It has a polymorphic structure; in some cases, light polymorphic cells, pseudoepithelial glandular formations and cysts predominate; in others, fibroblast-like atypical cells and collagen fibers, as well as tendon-like structures.
Malignant mesothelioma develops in the peritoneum, less often - in the pleura and cardiac membrane. Constructed of atypical large cells with vacuolated cytoplasm, tubular and papillary structures are often found (epithelial mesothelioma).
Osteosarcoma (osteogenic sarcoma)- malignant bone tumor. Constructed from osteogenic tissue, rich in extremely atypical cells -
 kami of the osteoblastic type with a large number of mitoses, as well as primitive bone. Depending on the predominance of bone formation or bone destruction, there are osteoblastic And osteolytic form osteosarcomas.
kami of the osteoblastic type with a large number of mitoses, as well as primitive bone. Depending on the predominance of bone formation or bone destruction, there are osteoblastic And osteolytic form osteosarcomas.
Chondrosarcoma It is distinguished by polymorphism of cells with atypical mitoses, a chondroid type of interstitial substance with foci of osteogenesis, mucus formation, and necrosis. Characterized by slow growth and late metastases.
Tumors of melanin-forming tissue
Melanin-forming cells of neurogenic origin (melanocytes) can be the source of tumor-like formations called nevi and true tumors - melanomas.
Nevi found in the skin, often on the face, body in the form of bulging dark-colored formations. There are several types of nevi, of which the most important are: borderline; intradermal; complex (mixed); epithelioid, or spindle cell (juvenile); blue. Borderline nevus represented by nests of nevus cells at the border of the epidermis and dermis. Intradermal nevus, the most common type, consists of nests and strands of nevus cells that are located only in the dermis. Nevus cells contain a lot of melanin. Multinucleated giant nevus cells are often found. Complex nevus has features of both borderline and intradermal (mixed nevus). Epithelioid (spindle cell) nevus found on the face mainly in children (juvenile nevus), consists of spindle cells and epithelioid cells with light cytoplasm. Characteristic are multinucleated giant cells, reminiscent of Pirogov-Langhans cells or Touton cells. There is little or no melanin in the cells. Nevus cells form nests both at the border with the epidermis and in the thickness of the dermis. Blue nevus occurs in people aged 30-40 years in the dermis, more often in the area of the buttocks and limbs. It has the appearance of a nodule with a bluish tint and consists of proliferating melanocytes that can grow into the subcutaneous tissue. In structure, a blue nevus is close to melanoma, but is a benign neoplasm and only rarely relapses.
Melanoma(melanoblastoma, malignant melanoma) is a malignant tumor of melanin-forming tissue, one of the most malignant tumors with a pronounced tendency to metastasize. It develops in the skin, pigment membrane of the eye, meninges, adrenal medulla, and rarely in the mucous membranes. It is possible to develop melanoma from a nevus. Most melanomas are localized in the skin of the face, limbs, and torso. Melanoma may appear as a brown spot with pink and black spots. (superficial spreading melanoma), blue-black soft nodule or plaque (nodular form of melanoma). It consists of spindle-shaped or
 Rice. 122. Melanoma
Rice. 122. Melanoma
lymorphic, ugly cells (Fig. 122). In the cytoplasm of most of them, yellow-brown melanin is found. Sometimes they meet non-pigmented melanomas. The tumor contains many mitoses, and there are areas of hemorrhage and necrosis. When a tumor disintegrates, a large amount of melanin and promelanin is released into the blood, which can be accompanied by melaninemia and melaninuria. Melanoma early produces hematogenous and lymphogenous metastases.
Tumors of the nervous system are very diverse, as they arise from different elements of the nervous system: central, autonomic, peripheral, as well as the mesenchymal elements that make up this system. They may be more or less mature, i.e. benign And malignant. However, being localized in the brain or spinal cord, they are essentially always malignant, since even with slow growth they put pressure on vital centers and cause disruption of their functions. Tumors of the central nervous system are divided into neuroectodermal and meningovascular (Table 10).
Table 10. Tumors of the nervous system and meninges

 Neuroectodermal tumors
Neuroectodermal tumors
Neuroectodermal (neuroepithelial) tumors The brain and spinal cord are built from derivatives of the neuroectoderm. They are more likely than tumors of other organs to be of dysontogenetic origin, i.e. develop from residual accumulations of precursor cells of mature elements of the central nervous system, and their histogenetic identity is sometimes established with great difficulty. More often, the cellular composition of tumors corresponds to certain phases of development of neuronal and glial elements of the nervous system. Neuroectodermal tumors include: astrocytic; oligodendroglial; ependymal and choroidal epithelial tumors; neuronal; poorly differentiated and embryonic (see Table 10). Malignant neuroectodermal tumors metastasize, as a rule, within the cranial cavity and extremely rarely to internal organs.
Astrocytic tumors
Astrocytic tumors(gliomas) are divided into benign - astrocytoma and malignant - astroblastoma (malignant astrocytoma).
Astrocytoma- the most common of neuroectodermal benign tumors, develops from astrocytes. Observed at a young age, sometimes in children; localized in all parts of the brain. The diameter of the tumor is 5-10 cm, it is not always clearly demarcated from the surrounding brain tissue, it has a uniform appearance on the section, and cysts are sometimes found. The tumor is poor in blood vessels and grows slowly.
There are three histological types of astrocytomas: fibrillar, protoplasmic and fibrillar-protoplasmic (mixed). Fibrillary astrocytoma rich in glial fibers arranged in the form of parallel running
 Rice. 123. Astrocytoma
Rice. 123. Astrocytoma
bundles, contains few cells such as astrocytes (Fig. 123). Protoplasmic astrocytoma consists of process cells of varying sizes, similar to astrocytes, and the processes form dense plexuses. Fibrillar-protoplasmic (mixed) astrocytoma characterized by a uniform arrangement of astrocytes and glial processes.
Astroblastoma(malignant astrocytoma) is characterized by cellular polymorphism, rapid growth, necrosis, and metastases along the cerebrospinal fluid pathways. Rarely seen.
Oligodendroglial tumors
Among oligodendroglial tumors There are benign ones - oligodendroglioma and malignant ones - oligodendroglioblastoma. Oligodendroglioma has the appearance of a focus of homogeneous gray-pink tissue. Constructed of small round or spindle-shaped cells, characterized by small cysts and calcareous deposits. Oligodendroglioblastoma (malignant oligodendroglioma) characterized by cellular polymorphism, an abundance of pathological mitoses, and the appearance of foci of necrosis.
Ependymal and choroidal epithelial tumors Among these tumors, ependymoma and choroid papilloma are benign, and ependymoblastoma and choroid carcinoma are malignant.
Ependymoma- glioma associated with the ependyma of the ventricles of the brain. It looks like an intra or extraventricular node, often with cysts
and foci of necrosis. Accumulations of unior bipolar cells around vessels (pseudorosettes) and cavities lined with epithelium (true rosettes) are typical.
Ependymoblastoma- malignant variant of ependymoma (malignant ependymoma). It is characterized by pronounced cellular atypia. In adults it may resemble glioblastoma, and in children it may resemble medulloblastoma. It grows quickly, infiltrating surrounding tissues and metastasizing through the cerebrospinal fluid system.
Choroid papilloma (choroid papilloma)- papilloma from the epithelium of the choroid plexus of the brain. It looks like a villous node in the cavity of the ventricles of the brain (Fig. 124), consists of numerous villous growths of cubic or prismatic epithelial cells.
Choroid carcinoma (malignant choroid papilloma) has the appearance of a node, is located in the ventricles, and is connected to the choroid plexus. Constructed from anaplastic integumentary cells of the choroid plexus (papillary cancer). Rarely seen.
Neuronal tumors
TO neuronal tumors include ganglioneuroma (gangliocytoma), ganglioneuroblastoma (malignant gangliocytoma) and neuroblastoma.
Ganglioneuroma (gangliocytoma)- a rare benign tumor, localized in the area of the bottom of the third ventricle, less often - in the cerebral hemispheres. Constructed of mature ganglion cells, their clusters are separated by bundles of glial stroma.
Ganglioneuroblastoma- malignant analogue of ganglioneuroma (malignant gangliocytoma)- an extremely rare tumor of the central nervous system. Differs in cellular polymorphism, similar to malignant glioma.
Neuroblastoma- a rare, highly malignant brain tumor that occurs in children. Constructed of large cells with a vesicular nucleus and numerous mitoses; the cells grow in the form of a syncytium, with many thin-walled vessels.
 Rice. 124. Choroid papilloma
Rice. 124. Choroid papilloma
Poorly differentiated and embryonal tumors
These include medulloblastoma and glioblastoma. Medulloblastoma- a tumor built from the most immature cells - medulloblasts, and therefore is particularly characterized by malignancy; its most common location is the cerebellar vermis. Occurs mainly in children (see. Diseases of childhood).
Glioblastoma- malignant, the second most common brain tumor after astrocytoma. It occurs more often at the age of 40-60 years. Localized in the white matter of any part of the brain. It has a soft consistency and a motley appearance on the section due to the presence of foci of necrosis and hemorrhages; its boundaries are unclear. It is built from cells of different sizes, differing in different shapes of nuclei, their size and chromatin content. There is a lot of glycogen in cells. Pathological mitoses are frequent: the tumor grows rapidly and can lead to the death of the patient within several months. Metastases develop only within the brain.
Meningovascular tumors
Tumors arise from the membranes of the brain and related tissues. The most common among them are meningioma and meningeal sarcoma.
Meningioma- a benign tumor consisting of cells of the pia mater. In cases where meningioma is built from arachnoid endothelium - the integumentary cells of the arachnoid membrane, they speak of arachnoidendothelioma. The tumor has the appearance of a dense node associated with the hard, less often soft, meninges (Fig. 125); built of endothelial-like cells, closely adjacent to each other and forming nested clusters. Cells often form microconcentric
 Rice. 125. Meningioma
Rice. 125. Meningioma
structures (meningotheliomatous arachnoidendothelioma); lime can be deposited in these structures, leading to the formation of so-called psammoma bodies. Meningioma can be built from bundles of cells and connective tissue fibers - fibrous arachnoidendothelioma.
Meningeal sarcoma- a malignant analogue of meningioma. Histologically, it resembles fibrosarcoma, polymorphic cell sarcoma, and diffuse meningeal sarcomatosis.
Tumors of the autonomic nervous system develop from different maturity of ganglion cells (sympathogonia, sympathoblasts, ganglioneurocytes) of the sympathetic ganglia, as well as from cells of non-chromaffin paraganglia (glomus), genetically related to the sympathetic nervous system. These include benign tumors - ganglioneuroma, benign non-chromaffin paraganglioma (glomus tumor, chemodectoma) and malignant tumors - ganglioneuroblastoma, sympathoblastoma (sympathogonioma) and malignant non-chromaffin paraganglioma (chemodectoma). Many of these tumors have been described previously.
Benign nonchromaffin paraganglioma (chemodectoma) its morphological characteristics are similar to tumors of the APUD system (apudomas), capable of synthesizing serotonin and, less commonly, ACTH. The tumor can reach large sizes, especially retroperitoneal ones. The most characteristic are the alveolar or trabecular structure, a large number of sinusoidal type vessels.
Malignant nonchromaffin paraganglioma (chemodectoma), which is rare, is distinguished by cellular polymorphism, infiltrating growth and lymphohematogenous metastasis. Sympathoblastoma (sympathogonoma)- an extremely malignant tumor, usually found in young children (see. Diseases of childhood).
Tumors of the peripheral nervous system arise from nerve sheaths. These include benign tumors - neurilemmoma (schwannoma), neurofibroma, as well as neurofibromatosis (Recklinghausen's disease) and malignant tumors - malignant schwannoma, or neurogenic sarcoma.
Neurolemmoma (schwannoma) built of spindle-like cells with rod-shaped nuclei. Cells and fibers form bundles that form rhythmic, or “palisade” structures: alternating sections of parallel nuclei (nuclear palisades, Verocai bodies) with sections consisting of fibers (Fig. 126). Neurofibroma- tumor associated with nerve sheaths. Consists of connective tissue with an admixture of nerve cells, bodies and fibers. Neurofibromatosis (Recklinghausen's disease)- a systemic disease characterized by the development of multiple neurofibromas, which are often combined with various
developmental defects. There are peripheral and central forms of neurofibromatosis.
Malignant neurilemmoma (neurogenic sarcoma)- a rare tumor. It is characterized by sharp cellular polymorphism and atypia, the presence of multinuclear symplasts and “palisade” structures.
Tumors of the blood system
Tumors of the blood system divided by systemic, or leukemia, and regional, or malignant lymphomas (cm. Diseases of the blood system).
Teratomas
Teratomas(from Greek teratos - monster, deformity) develop on
soil is the detachment of one of the blastomeres of the egg and may consist of one or more tissues. Teratomas are mature, benign tumors, but they can become malignant, then a malignant tumor develops - teratoblastoma(cm. Diseases of childhood).









